Anxiety disorders are among the most prevalent mental disorders in childhood and adolescence (
1,
2) and are associated with significant psychosocial impairment (
3). Although efficacious treatments exist (e.g., cognitive-behavioral therapy; CBT), at best about 60% of youths with anxiety respond to these treatments (
4). Clinical neuroscience has helped identify neural markers that differentiate treatment responders from nonresponders and could lead to neurobiological targets for future individualized treatment. Some of these neural markers even appear to predict treatment response better than behavioral and clinical measures (
5). Most work in anxious youths has focused on threat-related brain function, but growing evidence for the importance of reward in the pathophysiology and treatment of anxiety suggests the potential involvement of reward circuitry in treatment response.
Existing work on neural correlates of treatment response in anxious youths (
6–
8) is based on the premise of pathological anxiety as a function of altered threat circuitry—primarily amygdala hyperresponsivity. However, emerging research suggests that aberrant reward circuitry functioning is also implicated in the pathophysiology of anxiety. High state anxiety is associated with hypersensitive behavioral response to rewards in adults (
9), and youths with anxiety and those at temperamental risk for anxiety exhibit heightened neural responses to reward in the striatum (
10–
12), especially when rewards are contingent on their behaviors (
12). One interpretation is that heightened reward responding reflects strong sensitivity to feedback. Underexplored, however, is whether variability in reward function among youths has implications for understanding differences in treatment response.
The extent to which reward responsivity is associated with treatment response also remains unclear despite the importance of reward in engagement with psychotherapy. Intact reward circuitry is thought to play a role in affiliative behavior (
13) and motivational processes (
14), as well as to reflect stable tendencies to respond to pleasant stimuli (
14). During CBT, youths with more intact reward circuitry may be more likely to respond to social and tangible rewards (e.g., praise, stickers), which therapists use for reinforcement in youths for behavioral progress, to encourage engagement, and to build treatment alliance. Stronger patient engagement and therapeutic alliances are linked to better anxiety treatment response (
15). Even in nondirective supportive therapy, robust reward response could be important for experiencing positive emotions in therapy and developing a strong patient-therapist relationship. Given its role in approach behaviors, striatal function could also contribute to the effectiveness of exposures, a key behavioral component of anxiety treatment in which patients approach feared stimuli. Successful exposures induce pride and mastery in the child and are associated with anxiety reduction (
16). Youths with greater striatal responsivity may be more likely to complete exposures and to find successful exposures gratifying and beneficial.
Three studies to date, two of which included a majority of participants with co-occurring major depressive disorder, reported conflicting findings on how neural response to reward predicts treatment outcome in anxiety disorders. In a study of 52 adults with anxiety and/or depression, improvement in anxiety with CBT was related to lower pretreatment reward positivity amplitude, an event-related potential component that indexes responses to reward (
17). Using a similar task, a recent study of 27 youths with anxiety disorders found that reward positivity at baseline predicted change in depressive symptoms with CBT but did not predict change in anxiety symptoms (
18). However, in a functional MRI (fMRI) study of reward processing in 13 adolescents with anxiety and/or depression, anxiety improvement during CBT was associated with greater baseline striatal response to monetary reward (
19). In addition to the possible confounding influence of depression, the studies used different methods (EEG versus fMRI) and the sample sizes of the adolescent studies were small. Thus, the role of reward function in treatment response in anxious youths remains unclear.
In this study, using a large sample of early adolescents with anxiety disorders, we tested the extent to which pretreatment function in reward circuitry (i.e., the medial prefrontal cortex [mPFC] and striatum) is associated with differential treatment response. We hypothesized that before treatment, treatment responders would show greater striatal responsivity and weaker mPFC responsivity to reward than nonresponders. This pattern of brain function would potentially reflect a more typical response to reward; to explore this, we also included a group of matched healthy volunteers in analyses. The majority of anxious youths enrolled in this study completed CBT for anxiety (the Coping Cat program) after the pretreatment fMRI scan; less than one-third of the sample completed a comparison psychotherapy, Child-Centered Therapy (CCT). Although not a primary aim of the study, we explored whether associations between pretreatment brain function and treatment response differed as a function of treatment type. We also explored potential mechanisms that might help explain the association between reward-related neural activity and treatment response, such as positive affect and therapy engagement.
Methods
Participants
Ninety-two treatment-seeking youths with anxiety completed a pretreatment fMRI scan before randomization to either CBT or CCT as part of a randomized controlled trial examining predictors of response to treatment for childhood anxiety disorders (
20). All participants had an IQ >70 and met diagnostic criteria for current generalized anxiety disorder, separation anxiety disorder, and/or social phobia. Exclusionary criteria included use of psychoactive medications (with the exception of stimulants, which were not exclusionary but could not be taken the day of the fMRI scan); current diagnosis of major depressive disorder, obsessive-compulsive disorder, posttraumatic stress disorder, or attention-deficit hyperactivity disorder combined type or predominantly hyperactive-impulsive type; or lifetime history of psychosis, autism spectrum disorder, or bipolar disorder.
Of the 92 patients who completed the pretreatment scan, MRI data were usable for 81 patients (43 females; mean age, 11.02 years [SD=1.53]), and of these patients, 72 completed posttreatment clinical assessments (for more information, see the online supplement).
Thirty-seven healthy youths, group-matched with the patients on age, sex, and IQ (20 females; mean age, 11.64 years [SD=1.68]) completed the same baseline fMRI protocol. Two additional healthy youths also completed the baseline fMRI protocol but were excluded because of excess movement during scanning (N=1) and an incidental finding (N=1).
Procedure
The study was approved by the university’s institutional review board. After a brief telephone screen, participants and their primary caregiver completed an initial assessment with an independent evaluator. Active signed primary caregiver consent and youth assent were obtained for all participants after they had received a description of the study. After the initial assessment, eligible participants with anxiety disorders underwent randomized assignment to CBT or CCT, in a 2:1 ratio. Although two treatment types were used, our goal in the present study was not to compare the two but to examine how reward circuitry function was related to psychosocial treatment in general. This is in line with the goal of the larger study, which was to understand specific predictors and mediators of treatment response to CBT, while including an active comparison treatment that could speak to the effects of psychosocial treatment in general. This explains the 2:1 ratio in group assignment. Previous research published on this sample suggests that the majority of youths who received CBT and CCT responded to treatment, although some additional benefits were seen for the CBT group at follow-up (
20,
21). Although not a primary aim, exploratory, non-hypothesis-driven analyses comparing the treatment types were conducted. The MRI scan was obtained about 2 weeks before treatment began (mean=14.7 days, SD=8.1). Clinical assessments were administered again after treatment. Healthy comparison youths did not undergo treatment but did complete the MRI scan and relevant clinical assessments.
Measures
Clinical assessments.
The Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) (
22) and the Pediatric Anxiety Rating Scale (PARS) (
23) were administered by the independent evaluator to each patient and his or her parent separately to establish DSM-IV diagnoses and assess anxiety severity, respectively. A total PARS score was created by summing items assessing severity, frequency, distress, avoidance, and interference over the previous week. Treatment response (yes/no) was defined as a reduction ≥35% in PARS score from pre- to posttreatment assessment (
24). Patients completed the Mood and Feelings Questionnaire (
25) to assess depressive symptoms (for use as a covariate). In the healthy comparison group, only the K-SADS-PL and the Mood and Feelings Questionnaire were administered.
Participants also completed the Positive and Negative Affect Schedule for Children (
26) for a baseline measure of positive affect. Halfway through treatment (session 8), all youths with anxiety completed the Therapeutic Alliance Scale for Children (
27) to measure therapy engagement. Additionally, in the CBT group only, therapists reported the total number of exposures completed and the degree to which youths faced their fears during exposures (on a 1–7 scale). These measures were used in exploratory analyses examining potential behavioral mediators that might explain the link between reward circuitry function and treatment response.
fMRI task.
The fMRI paradigm is a well-validated block-design card-guessing game (
28) that probes neural response to the receipt of monetary reward. Participants guessed via button press whether the value on a card was higher or lower than 5, then received visual feedback about whether they won or lost (+$1 for each win, −50 cents for each loss). Each trial lasted 7 seconds; participants were given 3 seconds to guess, followed by presentation of the “correct” answer (500 ms), followed by visual feedback (green up arrow indicating win, red down arrow indicating loss; 500 ms), followed by crosshairs presented for 3 seconds. Each block consisted of five trials. Three reward blocks (80% positive feedback) and three loss blocks (80% negative feedback) were interspersed with three sensorimotor control blocks. The sensorimotor control blocks consisted of neutral trials during which participants pressed a button in response to an X on the screen. Blocks were presented in a pseudorandomized order, with outcomes (win/loss) predetermined. Each block was preceded by a 2-second instruction to either “Guess Number” or “Press Button” (for control blocks). Each block thus lasted 37 seconds, with a total task length of 5.55 minutes.
Treatment.
Treatment was 16 sessions of either CBT (N= 50) or CCT (N=22). CBT was Coping Cat (
29), an empirically supported manual-based treatment for children with generalized anxiety disorder, separation anxiety disorder, and social phobia. During treatment, youths learn anxiety awareness and regulation strategies, design and complete exposures, and earn rewards (e.g., stickers, toys) for exposures. CCT is a manualized treatment drawing on principles from client-centered therapy adapted for this study (
20). It includes humanistic, nonspecific techniques, such as active listening, reflection, and empathy, but does not include problem solving, exposures, or a structured reward program.
fMRI Acquisition and Analysis
Scanning was performed in a Siemens 3-T Trio MRI scanner. Blood-oxygen-level-dependent (BOLD) functional images were acquired using a T2*-weighted reverse echo planar imaging sequence. Thirty-two 3.2-mm axial slices were acquired parallel to the anterior-posterior commissure line (TR=1670 ms, TE=29 ms, FOV=205 mm, flip angle=75°, matrix=64×64). Structural scans (MPRAGE; 176 1.0-mm axial slices, TR=2100 ms, TE=3.31 ms, FOV=256×208 mm, flip angle=8°, matrix=64×64) were acquired before the start of the fMRI task.
Whole-brain image analysis was conducted in SPM8. Functional volumes were corrected for slice-timing and spatially realigned to account for head motion. Participants with motion >3 mm (mean across volumes) were excluded from the analyses. This cutoff was chosen to maximize the size of the clinical sample. Linear trends over the run were removed. Temporal filtering with a high-pass filter of 128 Hz was applied. Realigned images were spatially normalized into standard space (Montreal Neurological Institute’s MNI-152 standard template), and voxels were resampled to be 2×2×2. Normalized images were spatially smoothed with a 6-mm full width at half maximum Gaussian filter.
Preprocessed data were analyzed using second-level random-effects models accounting for scan-to-scan and participant-to-participant variability. For each participant, main effects of the task at each voxel in the brain were calculated using a t-statistic, producing a statistical image for the contrast of interest: win>loss.
BOLD Response
A one-way analysis of variance (ANOVA) in SPM12 (assuming unequal sample variance) was used to examine baseline differences in BOLD activation between healthy youths, treatment responders, and treatment nonresponders. Given our primary interest in neural differences related to treatment response, planned comparisons were conducted in the context of the ANOVA to examine whether treatment responders differed from nonresponders and whether treatment responders and healthy youths as a combined group differed from nonresponders.
Additional sensitivity analyses controlled for baseline anxiety severity to test whether pretreatment neural differences between treatment responders and nonresponders reflect differences in pretreatment anxiety severity, and/or for baseline depression severity, to test whether findings reflect differences in depression levels between the three groups. Analyses were limited to the striatum and mPFC (
19) using a single mask (see Figure S1 in the
online supplement) constructed using WFU PickAtlas, version 3.0.5b. The striatal portion was a sphere with a 20-mm radius centered on Talairach coordinates x=0, y=10, z=−10. The mPFC portion was a sphere with a 25-mm radius centered on Talairach coordinates x=0, y=44, z=18, encompassing anterior portions of the cingulate gyrus and medial portions of Brodmann’s areas 9 and 10. This mask was chosen to be consistent with previous research using the same task and similar samples (
19,
28).
To correct for multiple comparisons in these analyses, we first estimated intrinsic smoothness of the masked functional data using AFNI’s 3dFWHM module, version 20.1.11, with the spatial autocorrelation function (acf) option (
30). These acf parameters were applied to AFNI’s 3dClustSim module. Simulation results (10,000 Monte Carlo simulations) revealed the number of voxels needed to meet a starting voxel-wise threshold of p<0.005 and a cluster threshold of p<0.05 within the mask. A minimum cluster size of 896 mm
3 was needed for correction within this region of interest. We also present results at a more conservative voxel-wise threshold of p<0.001, with a minimum cluster size of 280 mm
3 needed for correction.
Discussion
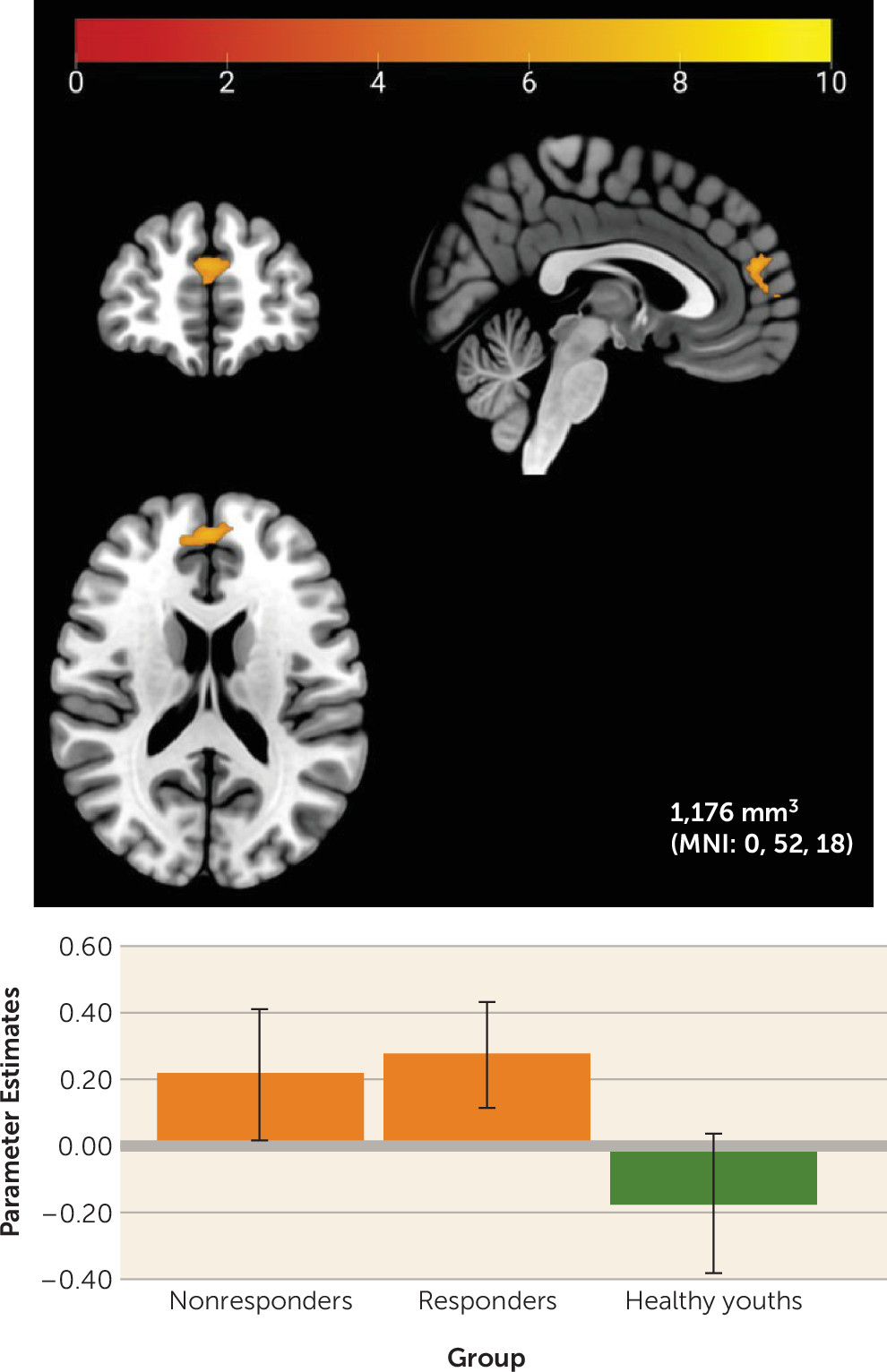
This study examined reward-related neural correlates of treatment response in youths with anxiety disorders. At baseline, both responders and nonresponders showed higher mPFC activation to reward relative to healthy comparison youths. Within the anxiety group, greater baseline response to monetary wins versus losses in a region of the left sgACC/NAcc was associated with successful treatment response. Findings were specific to neural activity during reward relative to loss feedback; neural activity during any feedback (reward and loss) relative to a control did not differ by anxiety status or treatment response.
Partially aligning with previous research showing heightened neural activation to rewards in anxious youths (
10), youths with anxiety demonstrated greater activation to rewards relative to nonanxious youths in the mPFC, a region unrelated to treatment response in this study. The mPFC activates to both anticipation and receipt of rewards (
33). This region also plays a crucial role in self-referential processing (
34), and some suggest that mPFC activity may help individuals weigh personal risks and benefits associated with their behavioral choices (
35), potentially through connections with the NAcc.
In this context, high mPFC activation to rewards in youths with anxiety could reflect heightened performance sensitivity during the guessing task, a task presented as one in which rewards are contingent on participants’ choices. Given the additional role of the mPFC in affect regulation and its connectivity with the NAcc, an additional interpretation could be that youths with anxiety are overregulating their initial striatal response to rewards. Notably, though, this finding did not survive a more conservative voxel-wise threshold and may have been driven by baseline differences between groups in depressive symptoms, aligning with previous research showing associations between heightened mPFC activity and depressive symptoms in youths and adults (
36,
37). Controlling for depressive symptoms in future research examining anxiety-related perturbations in reward responsivity is critical. The absence of striatal differences based on anxiety status could reflect this task’s focus on reward outcome and/or the mixed composition of both generalized anxiety disorder and social phobia in our sample. Most previous research shows anxiety-related striatal perturbations during reward anticipation (
10–
12), which we could not isolate in the present study. Research also suggests different patterns of neural reward function for youths with generalized anxiety disorder and youths with social anxiety disorder (
10,
38); combining youths with generalized anxiety disorder and social anxiety could mask these differential patterns. However, our study did not have adequate power to examine differences in neural activity by anxiety subtype.
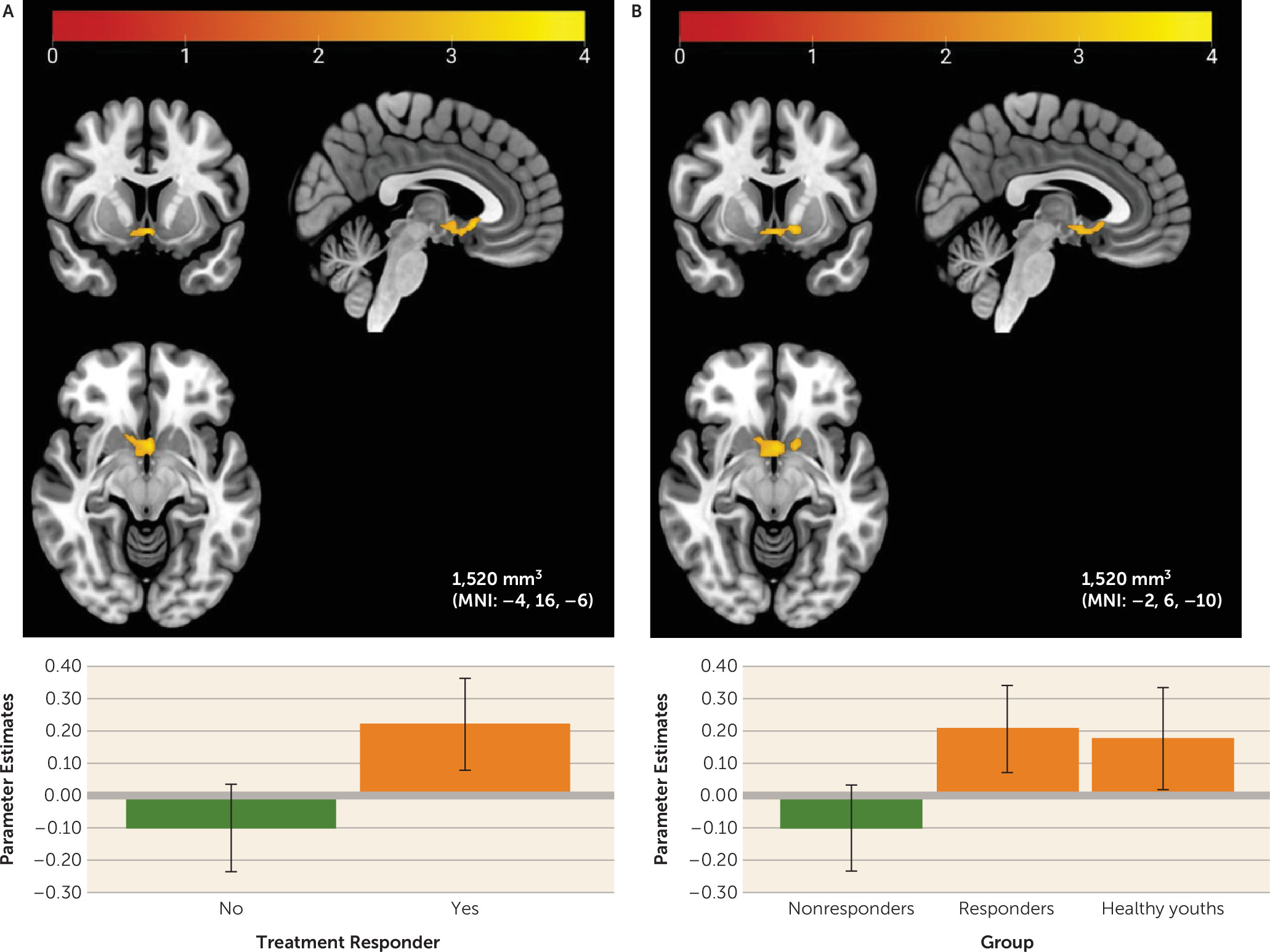
Planned comparisons also revealed that, relative to nonresponders, treatment responders showed higher baseline activity in the sgACC and NAcc, two sites of dopaminergic midbrain neuron projections (
39). This finding remained significant after controlling for baseline anxiety severity or depressive symptoms at a voxel-wise threshold of p<0.001. Healthy youths also showed a pattern of sgACC/NAcc activation similar to that of responders. The findings may suggest that responders exhibit healthier reward responding than nonresponders via more flexible phasic dopamine function, which allows youths with anxiety to engage effectively in treatment and improve in anxiety symptoms. Youths with this pattern of responding may be more motivated to complete exposures, more optimistic about treatment, more affiliative with the therapist, or more responsive to pleasant stimuli, which may increase engagement in therapy and support anxiety improvement (
15,
16). Positive associations between sgACC/NAcc activation to reward and self-reported positive affect could also suggest that robust reward function reflects greater capacity for positive affect during treatment and/or less anhedonia. Anhedonia has previously been associated with poorer treatment response in adolescents with depression (
40).
Future research may better address the question of how reward circuitry function affects the process of psychotherapy, as we had limited data to do this. Although we did not find that the quantity or quality of exposures during CBT mediated the association between neural activity and treatment response, these measures were recorded by the therapist. Examining how function in reward circuitry influences the child’s experience of therapy (e.g., how happy or proud a child feels after a successful exposure) may be important in future work. Future research could also explore whether the relationship between reward circuitry function and treatment outcome is related to learning processes. Reward, learning, and motivation are interrelated processes (
41), and learning is a core aspect of CBT (
42), particularly learning at the intersection of cognition, affect, and behavior. Finally, robust reward function could also be related to a less chronic form of anxiety, which we were unable to test but which should be considered in future research.
Interestingly, effects were not more pronounced for youths who received CBT relative to those who received CCT, a treatment that does not include exposures. Thus, reward responsivity may be important for response to psychosocial treatment in general, which is consistent with the notion that certain characteristics are generally associated with likelihood of improving with psychotherapy (
43). In addition, because CCT involves plentiful social support from the therapist, perhaps youths with greater neural sensitivity to reward generally are best able to benefit not only from tangible rewards and exposures in CBT, but also from the social rewards in both therapies. Indeed, basic neuroscience work suggests overlap in the neural substrates of social and monetary reward (
44). As youths receiving CBT and CCT were similarly likely to respond to treatment, common processes could underlie their similar efficacy. Of note, our study was underpowered to detect small to moderate differences between CBT and CCT responders and nonresponders (the post hoc power to detect an effect size of 0.25 was 0.55), and comparing neural mechanisms between treatments was not a goal of the larger study. Studies designed for this purpose, with larger samples balanced between treatment types, may be better suited to detecting meaningful differences.
The present findings align with previous neuroimaging work in adolescents (
19) but are inconsistent with research in adults showing that reduced reward positivity to reward predicts greater improvement in anxiety symptoms during CBT (
17) and recent work showing that pretreatment reward positivity to reward in anxious youths did not predict change in a continuous measure of clinician-rated anxiety severity during CBT (
18). Discrepant findings may be attributable to differences in the measurement of treatment response (i.e., categorical versus continuous; clinician-rated versus self-reported) or differences in the measurement of reward responsivity (i.e., fMRI versus EEG). Sample differences, including sample size and age, may also contribute to inconsistent findings. Future work could examine whether developmental differences, including heightened reward-related neural activity in adolescence, may help explain some inconsistencies.
This study benefited from a large sample size and rigorously applied evidence-based treatment, but it has several limitations. First, imbalanced group sizes may pose some concern, particularly regarding the planned comparisons (i.e., responders versus nonresponders). However, the ANOVA was run assuming unequal variance, and supplementary post hoc analyses also suggest that unequal variance did not drive the study findings (see the supplemental results in the
online supplement). Nonetheless, future research in this area should consider use of larger, more equally distributed groups (if possible) and/or more advanced prediction frameworks, such as connectome-based predictive modeling (
45,
46). While the present findings and interpretations should be viewed in light of the fact that several findings did not survive a more conservative voxel-wise threshold, the findings highlight key regions (e.g., NAcc, sgACC) that may be targeted a priori in future research and replication studies.
As mentioned, additional limitations of this study include low power to examine differences by anxiety subtype or treatment type, inability to model reward anticipation and outcome separately, and lack of extensive behavioral data to examine factors that might explain the link between reward-related brain function and treatment response. Future work examining reward processing disruptions in anxiety should attend closely to concurrent depressive symptoms, anxiety subtype, and reward task and methodology (e.g., EEG, fMRI). Tasks and methods that can reliably differentiate between neural activity during the anticipation of reward versus receipt of reward may be best suited for future studies, as anxiety-related disruptions in reward processing may be particularly potent during the anticipation of rewards (
10–
12). Future research should also examine whether reward responsivity predicts response to pharmacologic and other forms of treatment for anxiety in youths. Finally, replication and extension to methods less expensive than fMRI (e.g., EEG or behavioral measures) are critical next steps.
This work brings us closer to the possibility of improving existing treatments or developing personalized treatments guided by biology. The study findings, considered with previous research (
19), may signal a need for specific treatments designed for youths low in reward sensitivity. This work also holds promise for understanding the affective neuroscience of anxiety and has potential to inspire conceptual models of the role of reward in the etiology, pathophysiology, and course of anxiety.