Examining and Modulating Neural Circuits in Psychiatric Disorders With Transcranial Magnetic Stimulation and Electroencephalography: Present Practices and Future Developments
Abstract
THE ORIGIN OF TMS
TMS-EEG: THE TECHNIQUE
TMS-EEG TO EXAMINE NEURAL CIRCUITS IN HEALTHY INDIVIDUALS
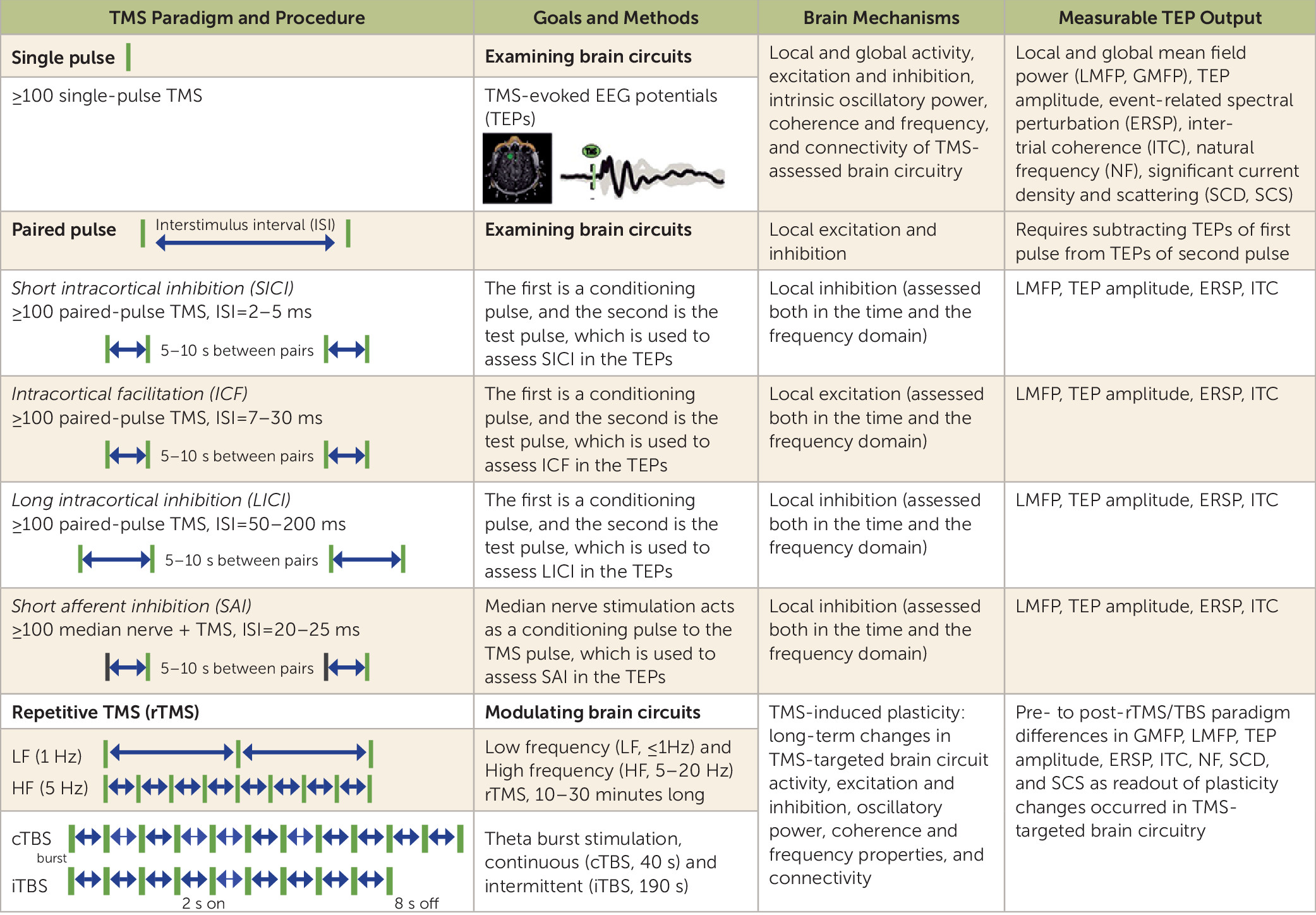
TMS-Assessed Cortical Excitation, Inhibition, and Oscillatory Properties
TMS as a Probe of Cortico-Cortical Effective Connectivity
rTMS and TBS as Neuromodulatory Paradigms for Neural Circuits in Healthy Individuals
rTMS- and TBS-Induced Changes in Neural Circuits Assessed With EEG and Behavioral Measures
TMS AS AN EXAMINATION AND A NEUROMODULATORY TOOL IN PSYCHIATRY
BOX 1. Transcranial magnetic stimulation (TMS): current practices in psychiatry
TMS Findings in Schizophrenia
TMS-Assessed Abnormalities of Cortical Excitation, Inhibition, and Oscillatory Activity in Schizophrenia
TMS as a Probe of Altered Effective Connectivity in Schizophrenia
rTMS and TBS as Interventions in Schizophrenia
TMS FINDINGS IN BIPOLAR DISORDER AND MAJOR DEPRESSIVE DISORDER
TMS-Assessed Abnormalities of Cortical Excitation, Inhibition, and Oscillatory Activity in Mood Disorders
TMS as a Probe of Altered Effective Connectivity in Mood Disorders
rTMS and TBS as Interventions in Mood Disorders
CHALLENGES AND OPEN QUESTIONS
CONCLUSIONS AND FUTURE DIRECTIONS
BOX 2. TMS: future developments in psychiatry
Employing TMS-Related EEG Measures to Elucidate Neural Circuit Dysfunctions in Order to Provide More Accurate Neural Targets for TMS-Based Interventions in Psychiatric Disorders
Acutely Modulating Neural Circuits With rTMS and TBS Paradigms and Examining Their Impact on Related Biological and Clinical Parameters to Better Inform Subsequent Neuromodulation-Based Treatment Interventions
Combining Neuroimaging, Neurophysiological, and Clinical Measures Related to TMS-Targeted Neural Circuits to Better Predict and Track Clinical Outcomes in TMS Clinical Trial Studies
Supplementary Material
- View/Download
- 129.12 KB
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

