Patients who experience difficulty initiating and maintaining sleep comorbid with a psychiatric disorder, most prominently depression, represent the largest subgroup of individuals with insomnia complaints.
1 Cognitive behavioral therapy for insomnia (CBTI) is an effective, nonpharmacological treatment of primary insomnia. It is recognized as a first-line treatment for insomnia by the National Institutes of Health (NIH) Consensus Statement
2 and the British Association of Psychopharmacology.
3 Emerging data furthermore indicate that CBTI is an effective treatment for chronic insomnia comorbid with depression and that it also leads to significant posttreatment improvements in depression.
4–6CBTI is comprised of behavioral components (stimulus control instructions, sleep restriction therapy, relaxation training, and other stress management techniques), a cognitive component (restructuring cognitions that interfere with sleep or adherence to the behavioral treatment elements), and recommendations that integrate both behavioral and cognitive elements (psychoeducation about sleep and sleep hygiene). Although most research studies have tested CBTI as a multicomponent package, therapists in clinical practice typically choose to implement and emphasize CBTI components that they consider most relevant to each patient’s clinical presentation.
The implementation of CBTI in patients with depression may require sensitivity to clinical features specific to depressive illness that may influence adherence and treatment outcome. For example, anhedonia and the tendency to try to sleep as a way to escape from emotional suffering may result in extended time in bed and thus contribute to the maintenance of insomnia. These depressive symptoms may also hinder adherence to behavioral components of CBTI by contributing to reluctance to get out of bed when unable to sleep, going to bed prior to becoming sleepy, and having difficulty adhering to a fixed rise time. The cognitive style and cognitive distortions inherent to depression may also impede resolution of insomnia among depressed patients. For example, the tendency to engage in ruminative worry, particularly while in bed, could impair the ability to fall asleep. Similarly, a biased attention to negative emotional stimuli in depression
7 may result in a greater tendency to catastrophize about the consequences of sleep loss. Both the tendency to ruminate and attentional biases would be expected to result in increased physiological and psychological hyperarousal and greater insomnia severity. Depressive symptoms, in this way, may hinder both response to and adherence with specific CBTI components. The present study thus aims to explore the impact of depression severity on insomnia outcomes (insomnia severity) and on adherence to various treatment components.
A few controlled studies document that sleep-focused treatments, including CBTI, result in overall reduction in depression severity.
4,8 However, because poor sleep is also one of the 9 defining symptoms of depression, it is difficult to discern the extent to which the observed reduction in depressive symptom severity is merely a reflection of improvement in a single symptom—sleep. For that reason the above studies removed the sleep item before examining the impact of sleep treatment on depressive severity. To shed further light on the complex relationship between sleep and depression we sought to evaluate the impact of CBTI on several non-sleep-related symptoms of depression, such as self-esteem, hopefulness, coping with stress, life enjoyment, perceived work productivity, and suicidal ideation.
The aims of the present study were three-fold: (1) to evaluate whether CBTI is more effective in improving insomnia severity and wellbeing and results in greater treatment satisfaction among patients with low compared to high depressive symptom severity; (2) to investigate the impact of CBTI on depressive symptoms; and (3) to assess the influence of depression symptom severity on adherence to specific CBTI treatment elements, thus informing the way CBTI may be best implemented when treating those with comorbid insomnia and elevated depressive symptom severity. The present study examined these three aims in a case replication series in which treatment-seeking chronic insomnia outpatients with and without comorbid psychiatric, medical, or sleep disorders received group CBTI in a clinic setting.
Methods
This study analyzes data gathered from patients who participated in group CBTI at the Stanford Sleep Disorders Clinic between 2000 and 2009. The protocol was pre-approved by the Institutional Human Subjects Review Board at Stanford University, and all procedures were HIPAA compliant. The protocol permitted use of de-identified, archival data collected prior to 2004. In subsequent years, all patients were invited to provide informed consent permitting use of their clinical data for research. This was a clinical community sample of outpatients who sought group therapy for chronic insomnia.
Participants
Prior to participation in the CBTI group treatment program, patients were evaluated by a physician or clinical psychologist board certified in sleep medicine or behavioral sleep medicine. Patients who were referred to the program presented with an initial complaint of insomnia. Because the rating of CBTI components was completed at the last treatment session, the sample consists only of treatment completers. Among those who consented and were included in the archival dataset, 301 completed the Beck Depression Inventory (BDI) at baseline at the last group session, when the posttreatment BDI was administered, and thus constituted the sample for this study. Those with comorbid psychiatric, sleep, or medical disorders, as well as those with concomitant use of sleep or other medications, were not excluded. Patients were not required to discontinue hypnotics prior to or during treatment. The sample for this study represents 45% of the total number of patients in the database who provided baseline BDI data. Reasons for missing data were: not attending the last treatment session, when posttreatment data were collected or not completing the posttreatment questionnaires. Patients not included in this study did not differ significantly on age, depressive symptom severity, or insomnia severity (all p-values > 1.0) from those who were included in the study sample.
Treatment
Treatment consisted of 7 (90-min) sessions of group CBTI delivered in a structured sequence. The first 5 sessions were delivered weekly, and the last 2 sessions occurred biweekly. Session 1 consisted of education about CBTI, orienting patients to the program, mini intakes, and goal setting. The remaining 6 sessions of this structured intervention consisted of psychoeducation about sleep and sleep hygiene (Session 2), stimulus control treatment and sleep restriction treatment (introduced in Session 3 and refined in Sessions 5 and 6), relaxation training (i.e., deep breathing exercises and other relaxation strategies for calming the mind prior to sleep initiation; Session 6), scheduled worry time (introduced in Session 3 and 4 and revisited in subsequent sessions), cognitive restructuring (discussed to some degree at each session), and relapse prevention (Session 7). Depressive symptoms were not directly addressed, except when they directly interfered with adherence to the behavioral components of CBTI. For example, when anhedonia made it difficult to adhere to the prescribed out of bed time, the therapist worked with the patient to identify and schedule morning activities to assist the patient in getting out of bed at the prescribed time.
Measures
Insomnia Severity Index
The ISI is a 7-item self-report scale that assesses subjective symptoms of insomnia, including the degree of distress and perceived impairment caused by the sleep complaint. Each item is scored on a 0 to 4 scale, with a maximum total scale score of 28.
9 A higher score represents greater insomnia severity. Scores > 11 are consistent with clinical levels of insomnia.
10 A recent validation study found that a reduction ≥ 7 points is consistent with partial remission by an independent rater, and a reduction ≥ 9 points is consistent with a clinical rating of marked improvement.
10Sleep Diary
Participants completed sleep diaries daily. Variables analyzed include: latency to sleep onset, time awake after sleep onset, total sleep time (derived from bedtime, wake time, and the preceding 2 variables), and sleep efficiency. Baseline and posttreatment values for each sleep variable were computed as the weekly average, respectively.
Beck Depression Inventory (BDI)
The BDI is a 21-item self-report inventory used to assess the severity of depressive symptoms. Participants are asked to indicate which statement best describes the way they have been feeling over the past week. Total scores on the BDI can range from 0 to 63, with higher scores reflecting greater levels of depressive symptoms. The BDI has yielded adequate reliability estimates, and has been well validated as a measure of depressive symptomatology.
11,12Treatment Satisfaction Scale (TSS)
The Consumer Report Treatment satisfaction scale
13 was adapted for insomnia in the present study to ask: “How much do you feel the insomnia treatment program has helped you in the following areas?” The areas listed included insomnia, energy level, work productivity, coping, life enjoyment, hopefulness, self-esteem, and mood. Each item was rated on a 5 point Likert scale, with 1 = Made things a lot better; 2 = Made things somewhat better; 3 = Made no difference; 4 = Made things somewhat worse; 5 = Made things a lot worse. All items on this measure were coded such that higher scores indicated greater symptom improvement. Overall treatment satisfaction was computed as the average score on all 7 items of the TSS, which provided an index of clinically significant improvement (Cronbach α = 0.86).
Treatment Components Adherence Scale (TCAS)
An author-constructed self-report scale was administered posttreatment to assess adherence to CBTI guidelines and the perceived helpfulness of treatment guidelines. Adherence to each therapeutic element was rated on a 0 to 3 scale as follows: (0) Followed rarely or not at all; (1) Followed occasionally; (2) Followed most of the time; (3) Followed consistently. To minimize spurious findings that may result from multiple testing for these variables, ratings for all therapeutic elements were broadly grouped into a
Behavioral Component and a
Cognitive Component.
Table 1 lists the therapeutic elements rated across these areas. The Behavioral Component consisted of stimulus control and sleep restriction guidelines and included the following 4 items: adhering to a fixed prescribed bedtime, getting out of bed when unable to sleep, using the bed only for sleep, and restricting the amount of time spent in bed (Cronbach α = 0.50). The Cognitive Component consisted of endorsement of 2 items: “Changing my expectations about sleep” and “Changing the way I think about not sleeping” (Cronbach α = 0.83).
Perceived helpfulness of each therapeutic element was rated as: Very helpful (3), Moderately helpful (2), Slightly helpful (1), Not helpful at all (0). Again, to minimize spurious findings that may result from multiple testing for these variables, ratings for all therapeutic elements were broadly grouped into four general areas, including the similar
Behavioral Component (Cronbach α = 0.70); a new set of items constituting a
Cognitive Component (consisting of the following four items: “not trying so hard to make sleep happen,” “accepting that sleep may not be forced,” “accepting that I may not get as much sleep,” and “learning that I can trust my own sleep system” (Cronbach α = 0.74); and two additional components, a
Nonspecific Component and a
Sleep Hygiene Component. The Nonspecific Component consisted of: “receiving input from group members,” “feeling that my problem is taken seriously,” “learning from group discussions,” “knowing that I can trust the treatment provider,” and “feeling hopeful that my insomnia will improve” (Cronbach α = 0.78). The Sleep Hygiene Component consisted of: not watching the clock at night, not exercising near bedtime, reducing caffeine or alcohol use, and not napping in the daytime (Cronbach α = 0.77).
Table 2 lists the therapeutic elements in each of these four areas.
Data Analysis
To test whether CBTI was more effective in reducing insomnia among patients with low depression severity compared to high depression severity, the sample was split using a validated BDI cutoff score for probable depressive disorder
12 as follows: a
high depression group (HiDep) consisted of patients whose baseline BDI was ≥ 14 (N = 120), and
a low depression group (LowDep) consisting of patients whose baseline BDI was < 14 (N = 181). This categorization was performed post hoc at the analysis stage. Thus, patients in both groups were not separated and received the same group treatment. A series of repeated measures ANOVAs were used to compare the change in ISI (the primary sleep outcome) and four diary-based sleep measures (latency to sleep onset, time awake after sleep onset, total sleep time, and sleep efficiency) from pre- to post-treatment, with Hi/LowDep as a between-subject factor. An independent
t-test was employed to compare the HiDep and LowDep groups on the TSS Insomnia item (secondary sleep outcome). Independent
t-tests were used to compare the HiDep and LowDep groups on overall treatment satisfaction (mean TSS items), which provided a measure of clinical significance, and enabled analysis of individual TSS items.
The differential effects on adherence to and perceived helpfulness of specific CBTI treatment components were assessed with a series of independent t-tests, comparing the HiDep and LowDep groups on adherence with and perceived helpfulness of specific CBTI treatment components as measured by the TCAS. To minimize type I error, we first compared group differences in each of the four treatment components. When a group difference for a given component emerged, it was followed by testing group differences in each of the specific therapeutic elements constituting this component.
To assess the impact of CBTI on depressive symptom severity, the analysis was restricted to the HiDep group. Pre- to post-treatment change in depression severity (BDI total scores, computed without the sleep item) was assessed with a paired t-test. The sleep disturbance item on the BDI (item 16) was deleted from the total BDI score to allow for assessment of CBTI effects on depressive symptom severity beyond disturbed sleep. Pre- to post-treatment change in suicidal ideation, assessed with BDI item 9, was analyzed using Wilcoxon Signed Ranks Test among the 65 individuals with a non-zero score on the BDI suicide item (9) at pretreatment.
Results
Complete data were obtained for 301 participants who had completed all of the above measures. Given that the TSS and TCAS were administered at the end of the study, only treatment completers were included in the present study. Participants (57.5% female), ranged in age from 21 to 88, with a mean age 49.6 (SD = 13.9) years and no difference between the HiDep and LowDep groups (p = 0.73). Data on marital status, ethnicity, and education level were not collected systematically. A total of 69.1% of the participants reported taking prescription medications for sleep; among those taking medications for sleep, the average number of days per week was 5.9 (SD = 2.04). The proportion of those reporting medication use for sleep did not differ significantly between the LowDep and HiDep groups (p = 0.50); however, those in the HiDep group tended to take these medications more often (5.86 vs. 5.33 days per week, p = 0.087). Hypnotic medication use was endorsed by 63.8% of participants, and an additional 5.9% reported taking other prescription medications with sedating effects, such as trazodone and quetiapine. Mean pretreatment scores were 19.7 (SD = 5.1) for ISI, 12.6 (SD = 7.3) for the BDI, and 0.26 (SD = 0.49, range 0-3) for BDI item 9 (suicide ideation).
Table 3 lists descriptive statistics for ISI at baseline and posttreatment.
Effects on Insomnia and Treatment Satisfaction
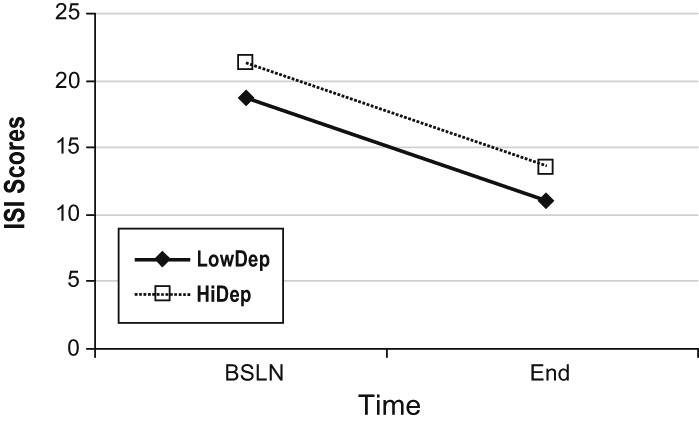
Figure 1 describes change in ISI scores in the 2 groups. There was no significant group (HiDep/LowDep) by time (pre- vs posttreatment) interactions (p = 0.61) on the change in ISI; but there were significant main effects for both time (p < 0.0001) and depression group (p < 0.001); this indicates that there was an overall reduction in pre- to posttreatment ISI score, and that the HiDep group had significantly higher ISI scores (p < 0.0001) throughout treatment. Thus, HiDep patients showed higher insomnia severity than LowDep patients at both pre- and posttreatment, yet benefited from group CBTI equally. Partial remission (≥ 7 point decrease in ISI) was attained by 59.8% of patients in the LowDep group and 60.4% of the HiDep group (Fisher exact p-value = 0.55). Marked improvement (≥ 9 point decrease in ISI) was attained among 39.0% of patients in the LowDep group and 43.8% in the HiDep group (p = 0.36).
Table 4 summarizes sleep diary data at baseline and posttreatment. A series of repeated measures analysis of variance revealed a significant decrease in latency to sleep onset and time awake after sleep onset, and a significant increase in sleep efficiency, with all p-values < 0.0001; no significant change in total sleep time (p = 0.22) was observed. There was no main effect for group or group × time, with all p-values > 0.2. Use of medications for sleep was reduced from 69.7% at baseline to 50.9% at the end of treatment, with no statistically significant difference between the groups (p = 0.24). Among those who used medications for sleep, the number of days medications were used dropped from 5.9 days per week at baseline to 5.5 at posttreatment, again with no significant group difference (p = 0.29). There were no significant differences between groups on TSS total score or individual items (all p-values > 0.14). The majority of participants reported that treatment made symptoms somewhat or a lot better (91% for insomnia, 65% for energy level, 59% for work productivity, 72% for coping, 68% for life enjoyment, 75% for hopefulness, 57% for self-esteem, and 60% for mood).
Adherence to and Perceived Helpfulness of CBTI Components
HiDep patients reported following the Behavioral Component to a lesser extent than LowDep patients (p = 0.004). Of the 4 items defining the Behavioral Component, HiDep participants reported poorer adherence to a fixed rise time (p < 0.0001) and to decreasing amount of time spent in bed (p = 0.016) compared to LowDep patients. In contrast, groups did not significantly differ in the extent to which they endorsed the Cognitive Component (p = 0.07); but patients in the HiDep group reported lower change in their expectations about sleep (p < 0.05).
Table 5 summarizes the extent to which each of the treatment elements were followed. Greater adherence to the Cognitive and Behavioral Components were associated with lower posttreatment ISI scores (r = −0.34, p < 0.0001; and r = −0.27, p = 0.002, respectively)
Table 6 summarizes the perceived helpfulness of each of the treatment elements. There were no significant observed differences between HiDep and LowDep patients in the perceived helpfulness of the 4 treatment components (Behavioral, Cognitive, Nonspecific, and Sleep Hygiene Components; all p-values ≥ 0.38). The rank order of the perceived helpfulness of these 4 components (Nonspecific, Cognitive, Behavioral, and Sleep Hygiene) did not differ between the LowDep and HiDep groups.
Effects on Depressive Symptoms
There was a significant reduction in depressive symptom severity (BDI total score after excluding the sleep item) in the whole sample (p < 0.0001) from 12.54 (SD = 7.27) pretreatment to 7.91 (SD = 6.20) posttreatment. Among HiDep patients, depressive symptom severity decreased from 19.74 (SD = 5.33) pretreatment to 12.02 (SD = 6.73) posttreatment (p < 0.0001, Cohen’s d = 1.45). A Wilcoxon signed ranks test revealed that CBTI elicited a statistically significant change in suicidal ideation pre- to posttreament (Z = −5.925, p < 0.001). According to analyses of rankings, 40 participants endorsed higher pretreatment suicidal ideation scores (range 0-3) than following treatment; 1 participant endorsed a higher suicide score following treatment (range 0-1); and 247 participants showed no change in pre to posttreatment suicidal ideation symptoms (range 0-1). Among the 65 participants who scored > 0 on BDI Item 9 for suicide ideation, pretreatment scores on the suicide ideation item were 1.10 (SD = 0.35) at baseline and 0.45 (SD = 0.50) at the end of treatment (p < 0.0001; Cohen’s d = 1.83); 45% of patients with suicide ideation at pretreatment no longer endorsed it at the posttreatment. At baseline, 23% scored > 0 on the BDI suicide item (21 % scored 1, 2% scored 2 or 3); whereas at posttreatment only 10% scored 1 (0% scored above 1).
Table 3 provides descriptive statistics for BDI pre and posttreatment.
Discussion
The present study evaluated how depressive symptoms impact pre- to posttreatment outcome following CBTI, and conversely, the effects of CBTI on change in depressive symptoms. Our findings indicated that group CBTI works equally well among those with high versus low depression severity. This finding is consistent with findings from previous studies showing that individual CBTI alone
6 or in combination with escitalopram,
4 treatment as usual,
14 and a self-help version of CBTI alone
5 are efficacious in the treatment of comorbid insomnia and insomnia refractory to antidepressant therapy.
14 However, the present study is the first to directly compare the relative effects of group CBTI among patients with high versus low depression severity. Our finding pertains only to patients who complete treatment. This is an important limitation, because elevated symptoms of depression at baseline have been previously identified as risk factors for early termination from group CBTI.
15 Although the rate of reduction in insomnia was clinically equivalent across both groups, overall insomnia severity remained higher among those with greater depression severity throughout treatment. This is consistent with past clinical and epidemiologic research, which has found greater insomnia severity among patients with elevated depressive symptoms and among those with a depressive disorder.
6,16Adherence to Treatment
We observed that adherence to two Behavioral Component elements of CBTI, keeping a fixed rise time and restricting time in bed, and one Cognitive Component treatment element, changing expectations about sleep, were lower among patients with greater depression severity. These findings suggest that greater attention to these three aspects of CBTI may enhance its efficacy among individuals with depressive illness. Possible adaptations may include: (a) scheduled activities in the morning and in the evening to increase adherence to morning rise time and facilitate TIB restrictions; and (b) greater attention to cognitive factors, such as patients’ beliefs about sleep and insomnia. These recommendations are congruent with effective psychological treatments of depression, such as cognitive therapy for depression, cognitive behavioral therapy for depression, and behavioral activation therapy, which strategically target dysfunctional cognitions, inactivity, and anhedonia.
11,17-19 Previous recommendations for adaptation of CBTI when applied to insomnia comorbid with depression
20 were based largely on clinical understanding of major depressive disorder, including how its symptoms may negatively impact sleep-related behaviors and cognitions. Data from the current study suggests that incorporating behavioral activation and cognitive therapy techniques into CBTI may further improve outcome for patients with elevated depressive symptoms.
The Impact of CBT-I on non-Sleep Clinical Outcomes (TSS)
We found that CBTI leads to significant reduction in depressive symptom severity (excluding the sleep symptom) among those with high depressive symptom scores. Results also revealed that CBTI was associated with a significant symptom reduction in suicidal ideation among those who endorsed suicidal ideation at baseline. Results furthermore suggested that CBTI positively impacts several non-sleep related aspects of depression, including enjoyment, hopefulness, self-esteem, coping with stress, work productivity, mood, and energy levels; and these beneficial effects did not differ according to depression severity. Perceived improvements in enjoyment, hopefulness, self-esteem, coping with stress, mood, and suicidal ideation suggest that the effects of CBTI on depression extend beyond simply improving just its target (sleep), through yet unknown mechanisms. We propose that improvements in sleep and hence energy may contribute to hopefulness, ability to cope with stress, and increased productivity, and translate into improvements in self-esteem and mood. Though plausible, this possibility awaits direct testing. Our findings are consistent with emerging data on clinical improvements in general functioning, such as depressive symptoms and perceived general health among older adults recruited from the community and primary care.
21 This recent study and our current findings demonstrate that in clinic samples, CBTI offers beneficial effects on symptoms that are not directly targeted by the therapy itself. The improvement in depressive symptom severity we found is consistent with previous studies, most uncontrolled, that examined the effects of CBTI on depressive symptom severity. These studies are reviewed in a report from a workshop that evaluated whether the effective management of sleep disorders could reduce both concurrent depressive symptoms and the risk of developing subsequent depression.
22 Seven of the eight studies that were reviewed found that CBTI improved both insomnia and depressive symptoms. The exception is a study by Lichstein and colleagues who studied patients with insomnia secondary to medical and psychiatric conditions. This study found that, although CBTI was effective in reducing insomnia symptoms, it did not reduce depressive symptom severity.
23A growing body of research suggests that disturbed sleep confers elevated risk for suicidal behaviors.
24-27 Sleep complaints are in fact listed among the top 10 warning signs of suicide by the Substance Abuse and Mental Health Services Administration (SAMHSA). To our knowledge, this is the first investigation to show that a sleep-focused therapy leads to a significant decrease in suicidal ideation. Of note, the observed effect size for this finding was large (d = 1.83), suggesting that CBTI results in a clinically meaningful reduction in suicidal ideation. Unlike many other suicide risk factors (e.g., past suicide attempt), insomnia constitutes a
modifiable risk factor for suicide. Our findings suggest that CBTI could enhance suicide prevention among patients with insomnia—an assertion that needs to be tested in a randomized controlled fashion, using a more rigorous assessment of suicidal symptoms than the single-item assessment used in the current study. If proved effective at reducing suicide risk, sleep-based suicide prevention may be appealing to patients because sleep disturbance is less stigmatizing than depression.
Limitations
Several limitations should be noted in the present study. First, this investigation was not a controlled trial and assessed treatment completers only. Second, we evaluated adherence to individual CBTI treatment components based on retrospective self-ratings, rather than measures derived from prospective sleep diaries or actigraphy. However, we assessed adherence with some important aspects of treatment that cannot be assessed with actigraphy or standard sleep diary entries, such as going to bed only when sleepy. We believe that the best way to assess adherence is through a combination of measures. Adherence to cognitive elements may be more difficult to qualitatively assess and quantify compared to behavioral treatment recommendations. Further refinement of the assessment of cognitive components is needed. Third, all sleep measures were subjective. With a shared method of variance, we cannot rule out the possibility that participants who reported better outcomes may have been more likely to report greater adherence to treatment. Lastly, our sample consisted of outpatients presenting to a sleep clinic for insomnia treatment. No exclusion criteria were used. Moreover, diagnosis of major depressive disorder was not assessed. While limiting internal validity, use of a clinical sample may be considered a strength of the study because it increases external validity and generalizability of study findings. Additional research is warranted to test if the results will extend beyond the presence of elevated depressive symptom severity—to patients with a comorbid depressive disorder and insomnia.
Conclusions
Insomnia patients with elevated depressive symptoms who completed treatment, pre- to post CBTI improvements in insomnia symptoms, depressive symptom severity, and other non-sleep related symptoms are similar to improvements seen among treatment completers with low depressive symptom severity. We also found that higher depression severity is associated with lower adherence to specific behavioral and cognitive therapeutic elements of CBTI.