Introduction
Major depressive disorder (MDD) is a highly prevalent disease with significant morbidity and mortality. Although there is a broad array of effective psychotherapeutic and psychopharmacological approaches, a significant number of patients do not respond despite aggressive management.
[1,2] When remission is achieved, relapse remains a significant problem, with the likelihood of remaining well at 6 months following our most effective acute interventions such as electroconvulsive therapy (ECT) as low as 46% despite aggressive relapse prevention strategies.
[3] Addressing this clinical need, new pharmacologic and nonpharmacologic approaches have been pursued recently for treatment-resistant depression (TRD), including daily left prefrontal transcranial magnetic stimulation (TMS).
Repeated daily left prefrontal cortex (DLPFC) TMS has been examined in psychiatry for more than a decade and its antidepressant activity has been extensively investigated. More than 35 randomized, placebo-controlled trials (RCTs), including over 1,200 MDD patients, have been conducted to investigate safety and efficacy of TMS as an acute antidepressant treatment.
[4] Meta-analyses of numerous RCTs indicate that administered to the left DLPFC daily for several weeks, TMS provides acute clinically significant antidepressant effects.
[5–11] As a result of a multicenter, industry-sponsored trial, the Food and Drug Administration approved TMS as a treatment for adult patients with nonpsychotic, unipolar TRD who failed to respond to a single antidepressant.
[12,13]While acute antidepressant benefits of TMS in TRD have been replicated in the recent NIMH-sponsored RCT with a validated active sham technique,
[14] few studies have assessed the durability of benefit following TMS. Dannon et al.
[15] followed a group of depressed patients who responded to acute treatment with ECT (
n = 20) or TMS (
n = 21). After 6 months, relapse rates were 20% in both groups. Patients on medications reported equally low and not significantly different scores in Hamilton Depression Rating Scale (HDRS) at 6-month follow-up. O’Reardon et al.
[16] reported that seven of 10 MDD patients received marked or moderate benefit from continuation TMS during follow-up over 6 months–6 years. Further, three of 10 patients stayed well with TMS alone. Fitzgerald et al.
[17] reported on 19 depressed TMS responders with relapses occurring from 6 to 11.6 months who experienced comparable benefit with TMS reintroduction. Maintenance of remission has also been investigated in 16 medication-free patients with TRD who initially had clinically significant antidepressant responses to a 10-day course of 10 Hz TMS and were followed for 4 years prior to and after completion of each TMS treatment course. Approximately 50% of patients sustained a clinically significant response to repeated courses of TMS despite the lack of adjuvant antidepressant medication. The mean interval between treatment courses was approximately 5 months, and the medication-free period ranged from 26 to 43 months. The duration of effect varied across patients, but benefits were sustained for a mean of nearly 5 months.
[18] Time to remission and maintenance of remission after TMS have been investigated by Cohen et al.
[19] in a large, retrospective, naturalistic study with 204 patients. After remission patients were followed up to 6 months: event-free remission was 75.3% at 2 months, 60.0% at 3 months, 42.7% at 4 months, and 22.6% at 6 months. Finally, Janicak et al.
[20] reported that the majority of patients who experienced acute clinical benefit with active TMS maintained this benefit over 24 weeks while on maintenance antidepressant monotherapy. Only 10 of 99 (10%) met criteria for relapse during this period compared with 16% (3/22) in the sham group. Of 38 out of 99 (38.4%), whose symptoms worsened after they achieved response and TMS was stopped, 32 (84.2%) regained mood stability with re-introduction of a short course of daily TMS.
In the current study, we examined persistence of benefit during a 3-month follow-up following acute treatment with TMS. Our primary outcome was the incidence of relapse during this period, defined by a total score on the HDRS-24 ≥20. We also explored the impact of demographic (e.g. age, gender) and clinical characteristics on long-term outcome (i.e. HDRS-24 scores at baseline and at the beginning of the follow-up) and whether a higher degree of medication resistance influenced long-term durability of clinical benefits.
Materials and Methods
Subjects
Patients were enrolled from October 15, 2004 through March 31, 2009 in a multicenter trial on efficacy, safety, and long-term durability of left DLPFC high-frequency and right DLPFC low-frequency TMS in TRD. The study was conducted at the Medical University of South Carolina (MUSC), Columbia University/New York State Psychiatric Institute, University of Washington, and Emory University. The IRB at each site approved the protocol, and all participants provided written informed consent.
One hundred ninety-nine patients met DSM-IV criteria for MDD, confirmed by Structured Clinical Interview for DSM-IV, with duration of the current episode ≤5 years and no active substance abuse or previous exposure to TMS. To qualify patients had to meet severity of illness criteria including a HDRS-24 ≥ 20
[21] and a moderate level of treatment resistance by the Antidepressant Treatment History Form
[22] (ATHF; insufficient clinical benefit to one to four adequate medication trials or intolerant to 3 trials). A complete description of the inclusion/exclusion criteria are described elsewhere.
[14]Study Design
The study included three phases: phase I was an RCT of high-frequency left DLPFC TMS
[14]; phase II was an open-label high-frequency left DLPFC TMS trial of patients who did not remit in phase I.
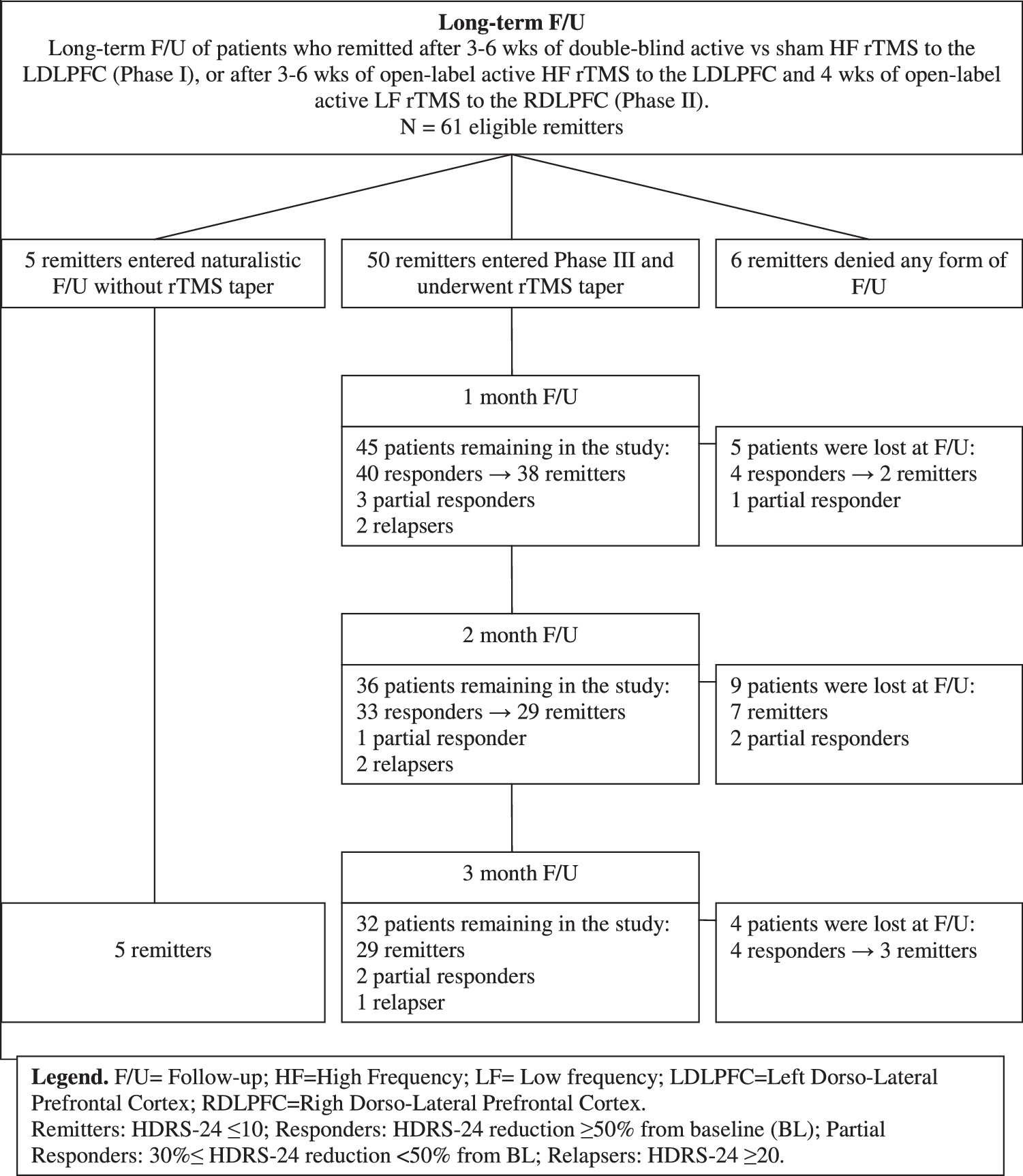
[23] Patients in phase II received up to 6 weeks of open-label high-frequency left DLPFC TMS. Patients who did not remit were switched to right DLPFC low-frequency TMS. Phase III was the long-term follow-up of patients who remitted in phase I or II and is the subject of this paper. A total of 61 subjects remitted after phase I and phase II and were eligible to enter phase III. Subjects were followed monthly for 6 months, but the retention from 3 to 6 months was too low (
n = 20) to allow meaningful analysis. Therefore, we report here the 3-month follow-up data.
Durability was assessed over 4 weeks of transition, which involved a gradual tapering of TMS (three sessions the first 2 weeks, two sessions the second 2 weeks) while consenting patients began open-label, continuation pharmacotherapy with an antidepressant plus optional mood stabilizer, and the rest were followed up in naturalistic fashion. All patients who entered phase III underwent TMS taper, regardless of whether they consented to be on continuation pharmacotherapy or not.
The investigators determined the pharmacological trials that were administered during the index episode at an adequate dose and duration to which patients did not respond, using ATHF criteria.
[24] The investigators also determined if patients did not respond to an adequate trial of venlafaxine, any tricyclic antidepressant, or any monoamine oxidase inhibitor. In this case, a different class of antidepressant (bupropion) was prescribed. The treatments preferred for TMS remitters was combination treatment with slow-release lithium carbonate and venlafaxine.
[25,26] If patients had already received an adequate combination of venlafaxine and lithium then first nortriptyline (if not used before) or then tranylcypromine would be used instead of venlafaxine, always combined with lithium. Over the first 10 days, while TMS was being tapered, the dosage of venlafaxine was increased to 300 mg/day if tolerated. Over the same period, the plasma level of nortriptyline was targeted at 125 mg/nL, but with levels between 50 and 150 mg/nL viewed as acceptable. Dosing of tranylcypromine was 60 mg/day as tolerated. The goal was to target 0.7 mEq/L plasma levels of lithium, with an acceptable range of 0.4−1.0 mEq/L, as tolerated. If patients met an exclusion criterion for treatment with lithium, lamotrigine was substituted, with the target being an oral dose of at least 200 mg/day. Lamotrigine dosing was slower, starting at 25 mg/day and increasing to 50 mg/day at 2 weeks. The dose was then increased to 100 mg/day in week 5 and 200 mg/day in week 6, monitoring closely for signs of rash and other side effects. The order of decision making was the determination of whether patients showed insufficient response to a trial involving venlafaxine and lithium during the index episode. If not, treatment with venlafaxine and lithium was recommended (or venlafaxine and lamotrigine for those with lithium exclusions or those declining lithium).
Patients, who did not consent to continuation pharmacotherapy with an antidepressant plus optional mood stabilizer, were followed in a naturalistic fashion until the end of the study.
TMS acute durability was assessed at the end of the TMS taper, and monthly during an 8-week period where patients continued either on the antidepressant plus optional mood stabilizer begun during the TMS taper phase, or on a different treatment prescribed by their personal physician. Symptoms were measured at each monthly visit by HDRS-24.
Statistical Analyses
Patients were classified to categories of response: remission (HDRS-24 ≤10); response (decrease ≥50% from phase I baseline HDRS-24); partial response (between 30 and 50% decrease from phase I baseline HDRS-24); relapse (defined as HDRS-24 ≥20). Nonparametric testing was applied to compare demographic and clinical data between remitters and relapsers. Specifically, the Mann–Whitney test was applied to evaluate group and time-dependent effects of TMS long-term antidepressant efficacy on HDRS-24. Pearson’s correlations were applied to examine the relationship between changes in depression scores with demographics and baseline clinical characteristics of remitters. All tests were conducted with two-sided significance levels (alpha = .05). All statistical analyses were performed using SPSS library, 17.0 version.
Results
Table 1 summarizes demographic and clinical characteristics of the 61 (32% out of 190 patients) remitters after phases I and II.
While both remitters in phase I and remitters in phase II who received on average 4 weeks of active TMS to the left DLPFC had failed one medication trial in the current episode, remitters in phase II who received on average 7 weeks of active TMS to the left DLPFC had failed two medication trials. Remitters to left plus right DLPFC TMS had the higher number of medication failures (n = 3) in the current episode. Overall, all remitters who finished 3-month follow-up received on average 6 weeks of active TMS and had failed two medication trials.
On the contrary, relapsers who were followed up to 3 months received on average 5 weeks of active TMS after failing two medication trials in the current episode. They significantly differed from remitters in a higher HDRS-24 total score at 1-month follow-up (after the TMS taper). Interestingly, four of five relapsers received active TMS and were those who regained remission by the end of follow-up.
Remitters in Phase I
In the phase I RCT there were 13 active and five sham remitters. The 13 (21.3%; M/F = 5/8; mean age = 47.7 ± 9.9) active remitters achieved remission after having failed 1.3 ± 0.8 medication trials in the current episode and having received 4.1 ± 1.4 weeks of active TMS. The five (8.2%; M/F = 1/4; mean age = 47.6 ± 12.6) sham remitters failed 2.2 ± 1.3 medication trials in the current episode and received 3.9 ± 0.8 weeks of sham. Both baseline HDRS-24 and level of resistance at the ATHF did not differ between the two groups. Sham remitters were included in the follow-up so as not to unblind the study.
Remitters in Phase II
From the open-label high-frequency left DLPFC TMS given in phase II, there were nine remitters who initially received active TMS in phase I and 13 who originally received sham in phase I. Nine patients (14.7%; M/F = 5/4; mean age = 50.4 ± 12.3) completed as remitters during phase II after failing 2.2 ± 1.6 medication trials in the current episode and having received 7.1 ± 1 weeks of active TMS. Thirteen (21.3%; M/F = 5/8; mean age = 48.1 ± 16.6) remitted during phase II after failing 1.4 ± 0.9 medication trials in the current episode and having received 7.4 ± 1.4 weeks of TMS, during which they were administered 3.9 ± 1.4 weeks of sham and subsequently 4.1 ± 1.5 weeks of active TMS. Although baseline HDRS-24 did not significantly differ between groups, the group that received active TMS in both the blinded and open-label phases had a higher, but not statistically significant, number of medication failures in the current episode. Finally, 21 (34.4%; M/F = 9/12; mean age = 49.7 ± 8.8) were classified as remitters after failing 2.8 ± 2.2 medication trials in the current episode and having received 10 ± 1.6 weeks of both high-frequency TMS on the left DLPFC and low-frequency TMS on the right DLPFC in phase II. Of these patients, 10 were randomized to active TMS and 11 to sham in phase I.
Remitters in Follow-up
Of 61 remitters after phases I and II, five entered naturalistic follow-up and declined enrollment in this study. They received outpatient care and were followed up at 3 months. Fifty remitters entered phase III. Thus, 55 of 61 patients agreed to participate in some form of follow-up. Of the 50 patients who entered phase III, 45 returned for the 1-month follow-up visit, 36 for the 2-month follow-up, and 32 for the 3-month follow-up (see
Figure 1). Overall 18 patients dropped out during the 12-week follow-up period (12 in a state of remission, three as responders, and three as partial responders).
Three of five sham remitters were followed up to 3 months: two patients were still remitters, and one relapsed. Of the other two sham remitters, one returned for the 1-month follow-up visit and was still a remitter.
Each of the five patients (M/F = 2/3; mean age = 45 ± 14.4) who entered naturalistic follow-up met remission criteria after 3 months. This subgroup failed 1.8 ± 1.3 medication trials in the current episode and received 4.4 ± 1.5 weeks of active TMS.
Of the 32 patients who completed phase III, 29 (58%) maintained remission, two (4%) were classified as partial responders, and one (2%) relapsed at the 3-month final visit. This patient was a sham remitter.
The 29 remitters (M/F = 12/17; mean age = 50.7 ± 10.6) had failed 2 ± 1.8 medication trials in the current episode and received 6.4 ± 2.9 weeks of active TMS.
A lower number of failed medication trials in the current episode, and a higher HDRS-24 total score at baseline were associated with a greater clinical benefit at 3-month follow-up (R = .4, P = .009 and R = − .8, P = .000, respectively).
Relapsers in Follow-up
Of the 37 patients who completed in some form the follow-up, over the entire 12 weeks following phase I and II, five patients (M/F = 1/4; mean age = 57.4 ± 11.4) relapsed (relapse rate = 13.5%). This subgroup failed 2 ± 1.2 medication trials in the current episode and received 5.1 ± 4.2 weeks of active TMS. However, one was a sham remitter and relapsed at 3 months, while the other four patients, who had received active TMS, regained remission (one at 2 months, three at 3 months of follow-up) and, on average, the HDRS-24 of the five relapsers at 3 months was ≤10. The average time to relapse in all five patients after acute TMS was 7.2 ± 3.3 weeks.
Relapsers (n = 5) significantly differed from remitters (n = 29) in the HDRS-24 scores at 1-month follow-up (Mann–Whitney U = 29.5, P = .04).
Concomitant Treatments During 3-Month Follow-up
Ten remitters (34.5%), of whom two remitted after sham TMS, were administered venlafaxine maintained on an average dose of 204.5 ± 89.3 mg/day, and two (6.9%) were administered buproprion on an average dose of 300 mg/day; of them seven (one was a sham remitter) were on concomitant lithium maintained on an average dose of 471.4 ± 292.7 mg/day (average blood level of 0.35 ± 0.2 mEq/L) and four on lamotrigine maintained on an average dose of 200 ± 50 mg/day. Therefore, 11 of 29 (37.9%) remitters were on combination pharmacotherapy. Three remitters (10.3%) were on sleeping medications (two on an average dose of 10 mg/day of zolpidem and one on a dose of 1 mg/day of lorazepam) and 14 (48.3%) were on no medication. Of these, two were sham remitters.
Two relapsers (40%) were administered antidepressants, one was on venlafaxine maintained on a dose of 150 mg/day and the other on sertraline maintained on a dose of 50 mg/day; of them the first patient was on 300 mg/day of lithium as well. Two relapsers (40%) were on sleeping medications (one who had remitted after sham TMS was on a dose of 10 mg/day of zolpidem and one on a dose of 1 mg/day of lorazepam) and one (20%) was on no medication.
Discussion
This study examined the persistence of antidepressant benefit over 3 months in medicated and unmedicated unipolar depressed patients who had achieved remission during a RCT of the antidepressant efficacy of daily prefrontal TMS. While one third of the sample was lost to follow-up, our results demonstrate that most patients contributing observations experienced persistence of benefit from TMS followed by pharmacotherapy or no medication, with a low overall relapse rate of 13.5% (5/37).
Most patients (50/61) who experienced acute clinical remission from their TRD by TMS entered the 3-month follow-up with controlled pharmacotherapy or in naturalistic follow-up; and of these, 32 fully adhered to the protocol were seen monthly until the end of the study, and were put on concomitant medications (55.5%) or followed up naturalistically. Fifty-eight percent of adherent patients maintained remission, while 4% were classified as partial responders, and only 2% relapsed. Interestingly, along with the five patients who did not enter the TMS taper phase but agreed to be followed up naturalistically, the majority of the patients lost at follow-up (n = 12) were in a state of remission at their last visit. Of the others who were lost during follow-up, three were responders and three were partial responders.
For those who did relapse, the average time to relapse after acute TMS (n = 5) was 7.2 ± 3.3 weeks, so relapse appeared to be skewed to early in the follow-up phase, although the relapse was not immediate. However, four of those patients who relapsed regained remission within 3-month follow-up (on average after 11 ± 2 weeks from the beginning of the follow-up).
Our results suggest that number of failed medication trials and symptoms severity in the current episode are important factors related to outcome over a 12-week follow-up. While small sample size, high drop-out rates, and the nonrandomized nature of the follow-up prohibits formal statistical testing, this observation is in line with the results found by Lisanby et al.
[13] who identified that the level of treatment resistance and symptoms severity at entry are critical predictors for acute TMS benefit.
Notably, our results of relapsers who significantly differed from nonrelapsers in the HDRS-24 scores at 1-month follow-up suggest that the end of the TMS taper phase might be a critical time period. Long-term continuation treatment with TMS might be a useful approach to test in a structured fashion. So far preliminary data from case series report that delivering five sessions of daily TMS every fifth week kept one patient free from depressive episodes for a period of 12 months.
[27] Alternatively, one to two TMS sessions per week resulted in seven of 10 subjects experiencing either marked or moderate benefit in a period ranging from 6 months to 6 years.
[28] The same TMS schedule was used in a case of severe psychotic depression who reported a significant clinical improvement after acute TMS with a long-lasting antidepressant effect up to 14 months.
[29]An alternative strategy might be the use of continuation pharmacotherapy with an antidepressant medication or an antidepressant in combination with a mood stabilizer, which has been proven to be more effective than monotherapy after a successful course of ECT.
[30] In our sample about one third of the remitters were on combination pharmacotherapy, and the comparisons between the subgroup of remitters on medications (15/29) and the subgroup of remitters without medications (14/29) did not show significantly different clinical outcomes at 3-month follow-up.
A recent RCT
[20] that described the time course of continued benefit after 6 weeks of high-frequency TMS to left DLPFC by using as maintenance antidepressant monotherapy, found a relapse rate of 10.1%. These results are comparable to ours, with the difference that in our study the follow-up was mostly naturalistic in nature and shorter. Therefore, a RCT, including an antidepressant, antidepressant + mood stabilizer or placebo, should be carried out in order to test rigorously continuation pharmacotherapy strategies after a successful course of TMS in TRD.
Conclusion
Limitations of the study are the small sample size of patients who completed the 3-month follow-up and the relatively low medication levels of both antidepressants and mood stabilizers in those patients who consented to continuation pharmacotherapy. However, the subtarget dosing would serve to underestimate the durability of the treatment, thus we might expect more aggressive dosing to result in even lower relapse rates than those reported here.
These data provide descriptive evidence of reasonable long-term (3 months) durability of clinical benefits of acute TMS, and illustrate some strategies (i.e. TMS taper and post TMS pharmacotherapy) to maintain the acute benefit after a course of TMS. Future work with a higher rate of patient completion is needed to critically examine the true durability of TMS-induced antidepressant response, optimal treatment strategies for relapse prevention, and how to tailor these strategies for those most at risk for relapse.