Introduction
Drug delivery to the brain is limited by the blood-brain barrier (BBB), which prevents the entry of over 98% of small-molecule drugs [
1]. Substances that can cross the BBB via transmembrane diffusion generally have molecular masses under 400-500 Da and high lipid solubility [
1]. Vascular endothelial cells (ECs) can also transport substances such as glucose and amino acids through transporter complexes located on the luminal or abluminal surfaces of the ECs [
2], but have notably reduced endocytosis in the BBB compared to peripheral endothelia [
1].
An emerging strategy to temporarily and locally increase BBB permeability for targeted drug delivery is to use transcranial focused ultrasound (FUS) in conjunction with preformed, systemically circulating microbubbles (MB
s). In this approach, during FUS exposure, MBs oscillate in the vasculature and exert mechanical stresses on blood vessel endothelia, resulting in increased paracellular and transcellular transport. In addition to enhanced agent delivery [
3-
8], FUS+MB exposure has been shown to induce biological effects such as neurogenesis in the dentate gyrus [
9,
10], glial cell activation [
11], and a reduction in β-amyloid (Aβ) plaque load in transgenic mouse models of Alzheimer's disease (AD) [
12-
15].
The mechanisms by which FUS+MB BBB treatments clear Aβ pathology have yet to be fully elucidated. While FUS+MB BBB treatments have been shown to induce glial cell activation and phagocytosis of Aβ plaques [
11,
13], there is likely a saturation limit [
11]. In addition, microglia, the resident macrophages in the CNS, are poor phagocytes compared to peripheral immune cells, in part due to the lack of certain cell surface antigens that facilitate immune responses, such as major histocompatibility class II molecules and CD45 [
16]. Infiltration of peripheral blood monocytes following FUS+MB BBB treatments has been previously reported; however, such events were only observed coincident with vascular damage, such as clusters of erythrocyte extravasation [
17] or intracerebral hemorrhage [
18]. It is possible that peripheral immune cells have not been previously detected in FUS+MB BBB treatments that did not result in damage due to the small number of participating cells, and the limits of detection of the assays used. FUS+MB treatments delivered using a clinical imaging dose of MBs have been shown to result in an acute inflammatory response, characterized by an upregulation of pro-inflammatory genes, such as
Sele, Cxcll, and
Ccl3, at 6 h following treatment [
19,
20]. In addition, use of FUS+MB to increase blood-tumor barrier permeability has been observed to induce a mild and transient response in dendritic cells [
21]. It is hypothesized that a cellular aspect of acute inflammatory responses, specifically the recruitment of peripheral immune cells, would be induced by FUS+MB BBB treatments as well.
In this paper, the recruitment of peripheral immune cells induced by FUS+MB BBB treatments and their role in FUS+MB mediated Aβ plaque clearance was investigated. To observe the response of vasculature and cells to FUS+MB treatments in real-time, transgenic EGFP Wistar rats which harbour several fluorescent cell types were monitored in vivo using two-photon fluorescence microscopy (2PFM). This study reveals a previously unreported cellular component of the acute inflammatory response that results from FUS+MB mediated BBB treatments, which may be useful in informing future studies involving neuroinflammatory disease conditions (e.g. AD) or in consideration of using FUS+MB BBB treatments for drug delivery. This study is relevant as clinical trials involving the use of FUS+MB BBB treatments are currently underway (ClinicalTrials.gov identifier: NCT04118764).
Materials and methods
There were three arms to this study: (A) In vivo 2PFM monitoring of peripheral immune cell response to FUS+MB BBB treatments, (B) Identification and quantification of participating peripheral immune cells using immunohistochemistry, and (C) Investigation into the potential of the identified immune cells in assisting in FUS+MB mediated Aβ plaque clearance.
In vivo 2PFM Monitoring of Peripheral Immune Cell Response to FUS+MB BBB Treatments
Animal preparation
All animal procedures were approved and conducted in compliance with the Animal Care Committee guidelines at Sunnybrook Research Institute, Canada. Male and female EGFP Wistar rats [Wistar-TgN(CAG-GFP)184ys] (310-630 g) (
n = 15) were used in this study. Transgenic EGFP rats harbour the EGFP transgene and pCXN2 expression vector containing cytomegarovirus enhancer, chicken β-actin enhancer-promoter, and rabbit β-globin poly(A) signal [
22]. The GFP gene was designed to be expressed ubiquitously in this rat model, enabling monitoring of many different cell types [
23].
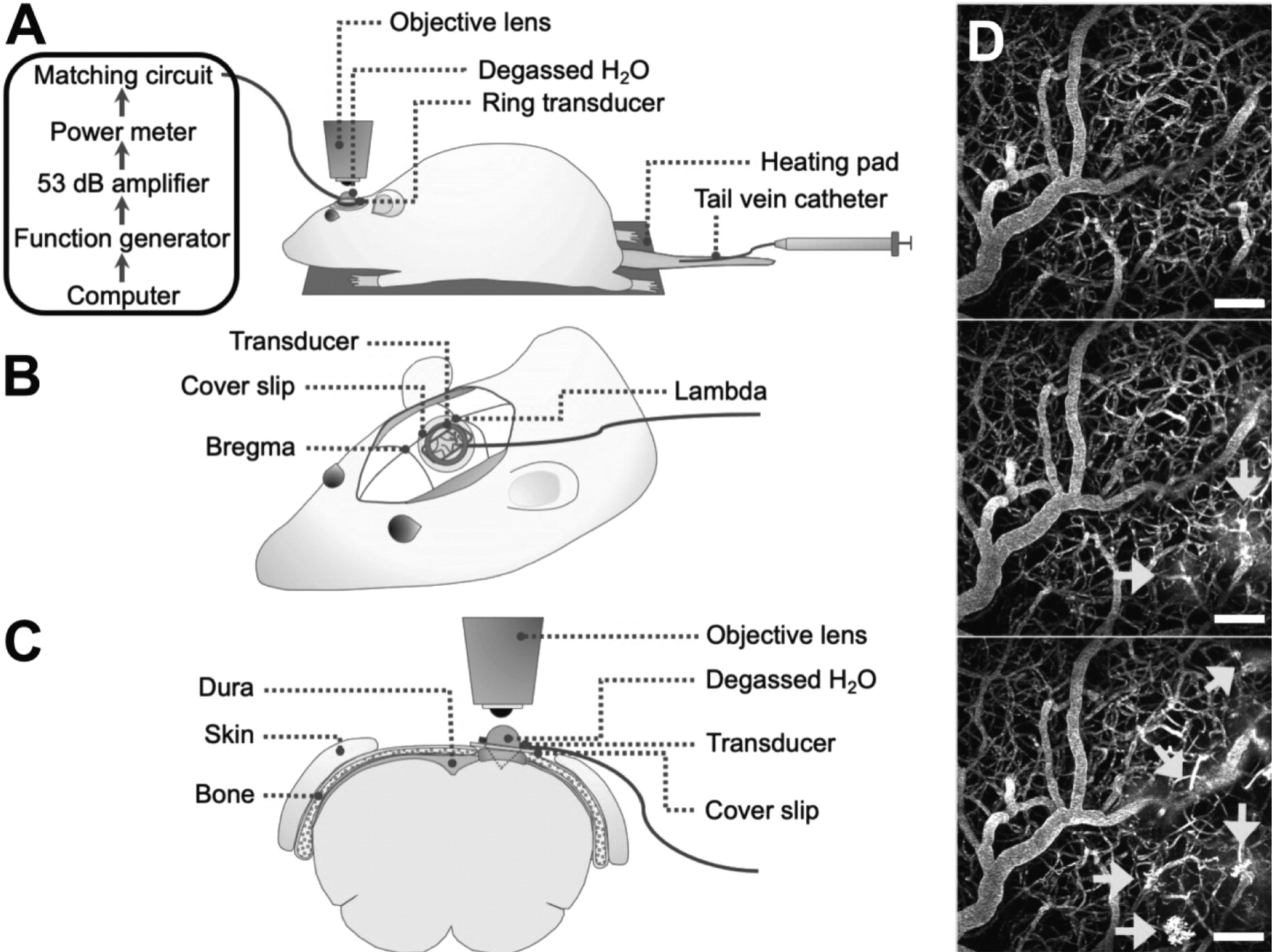
Rats underwent cranial window surgeries for acute 2PFM experiments (
Figure 1). For the creation of cranial windows, rats were anesthetized with isofluorane and a core body temperature of 37°C was maintained using a heating pad with a rectal thermistor (TC-1000; CWE Inc., USA). The rat head was secured in a stereotaxic frame and a cranial window was created in the parietal bone by removing a rectangular (∼ 3 × 4 mm) piece of bone using a dental drill. The dura was removed for optical clarity. Agarose gel was used to fill the removed portion of skull and dura for acoustic coupling, and a circular cover slip (5 mm diameter, no. 1 thickness; CS-5R, Warner Instruments, USA) was adhered to the cranial window using cyanoacrylate adhesive. The tail vein was cannulated with a 27 G catheter to enable intravenous injection of fluorescent dextrans and MB
s.Two-photon fluorescence microscopy
Imaging was performed on an FV1000MPE multiphoton laser scanning microscope (Olympus Corp., Japan) with an InSight DS tunable laser (Spectra-Physics, USA). A 25x water-immersion objective lens (XLPN25XWMP2, NA 1.05, WD 2 mm, Olympus Corp., Japan) was co-aligned with the ring transducer and water-coupled for imaging at lateral and axial resolutions of 0.994 μm/pixel and 2 μm/slice, respectively, and at an imaging speed of 2-8 μs/pixel for a maximum imaging duration of 4 h. Texas Red 70 kDa dextran (dissolved in PBS, 5 mg/kg; Invitrogen, Canada) was injected through a tail vein catheter for visualization of vasculature. EGFP cells and Texas Red dextran were excited at 900 nm. Fluorescent emissions were collected with photomultiplier tubes preceded by the following bandpass filters: 575-645 nm for Texas Red and 495-540 nm for EGFP.
After administration of Texas Red dextran, XYZT image stacks (XY: 0.994 μm/pixel, typically 512 × 512 pixels; Z: 2 μm/slice, 6-10 slices) were acquired to assess baseline immune cell dynamics. Regions-of-interest (ROIs) were centred on blood vessels that showed increased permeability following FUS+MB treatment. Imaging duration ranged from 2-4 h and was limited by increased noise from fluorescent cells reacting to the acute cranial window in the superficial layers of the cortex.
Focused ultrasound and microbubble treatment
An in-house manufactured lead zirconate titanate (PZT-4) cylindrical transducer (10 mm diameter, 1.5 mm thickness, 1.1 mm height) was coupled to a circular glass coverslip with cyanoacrylate adhesive, matched to a 50 Ω impedance and 0° phase load with a custom matching circuit, and driven at 1 MHz in thickness mode, producing a circular focal spot 1 mm beneath the coverslip [
24]. The transducer was air-backed with a droplet of degassed deionized water in the centre of the transducer to allow for use with a water-immersion objective lens (
Figure 1). The transducer was driven by a computer-controlled function generator (Agilent, Palo Alto, USA), amplified with a 53 dB RF power amplifier (NP Technologies Inc., Newbury Park, USA), and transmitted through an in-house power meter and matching circuit prior to reaching the transducer. Definity
TM MB contrast agent (diluted 1:10 v/v in saline, 0.04 ml/kg; Lantheus Medical Imaging, MA) was administered intravenously through the tail vein catheter prior to sonication. Sonications were delivered to one location in 10 ms bursts, 1 Hz PRF, 120 s total sonication duration, with estimated
in situ peak pressures of 0.28-0.55 MPa.
Vessel segmentation and permeability analysis
Two-photon fluorescence XYZT image stacks were analyzed in MATLAB (Mathworks, USA). The 575-645 nm channel (Texas Red dextran signal) in baseline images was corrected for GFP bleed-through, median filtered by 3 pixels in 3-dimensions, and contrast enhanced. Thresholding was then used to create a binarized vessel mask and vasculature was segmented.
Vessel leakage was assessed by applying the baseline mask to subsequent image stacks. Here, individual vessels with observable leakage were assessed by defining an ROI in 3D, such that the extent of leakage was captured without including adjacent vessels. For each ROI, fluorescence intensity in the intra- and extravascular regions was assessed over time and normalized to compartmental volume and baseline fluorescence, with the assumption that fluorescence intensity is proportional to concentration.
Spatiotemporal leakage was assessed by performing a Euclidean distance transform, creating a distance map from each extravascular pixel to the nearest vascular structure and removing boundary effects by truncating 20 pixels on all sides. Extravascular fluorescence was normalized to compartmental volume at each distance away from the nearest vessel, and to baseline fluorescence.
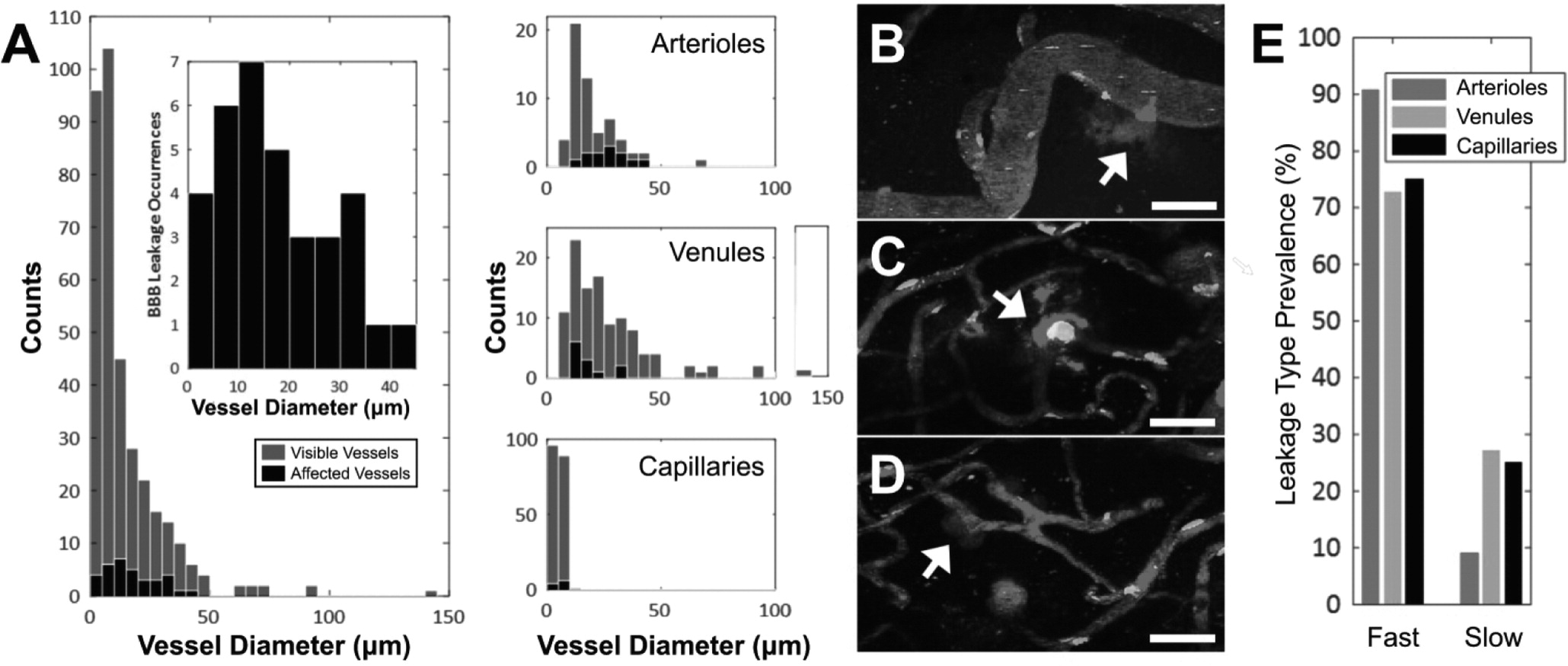
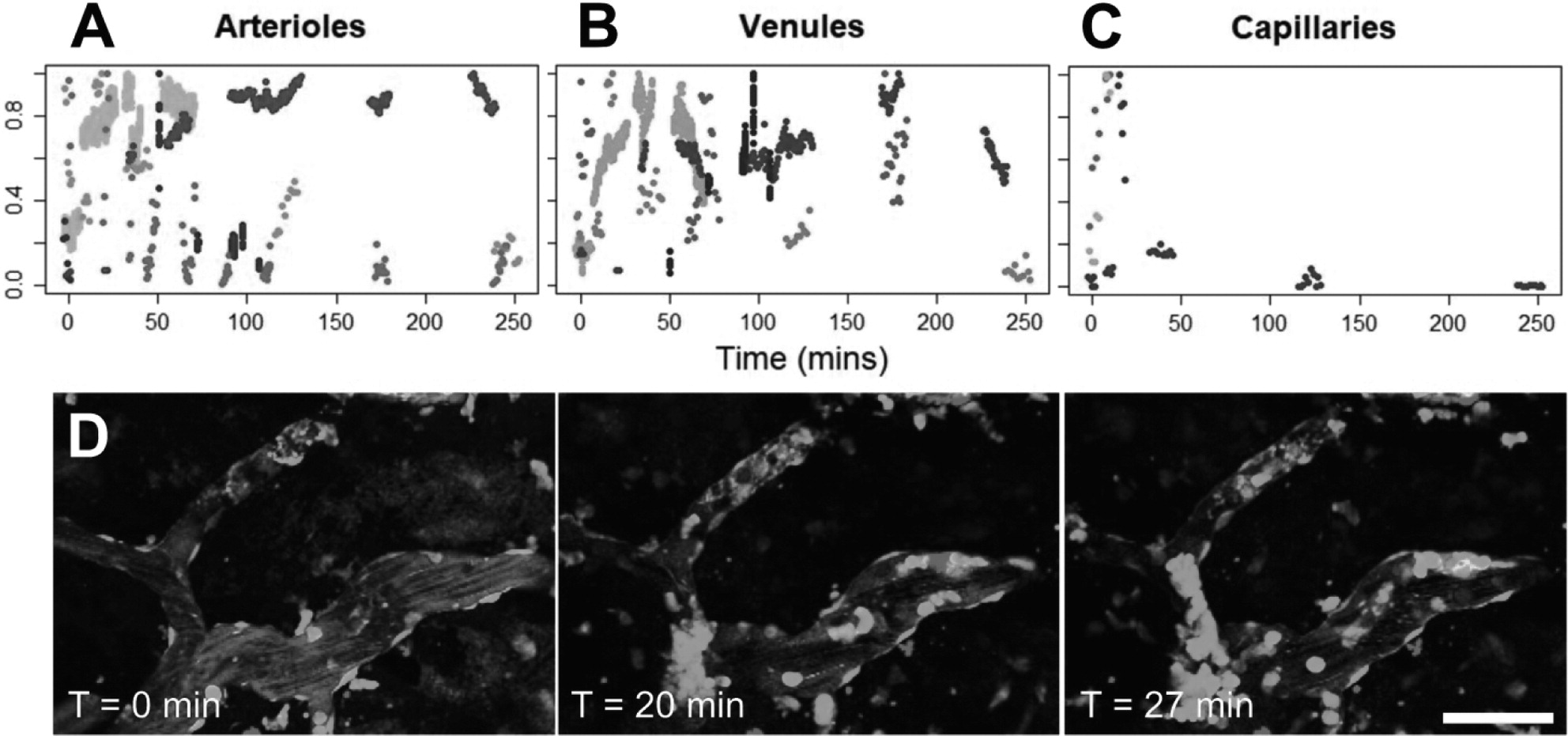
Vessel diameters were determined by taking the maximum length of the signal profile perpendicular to the local tangent. New vessels were defined whenever vessel branching occurred. Vessels were classified as arterioles, venules, or capillaries based on diameter, branching, and tortuosity patterns [
25,
26].
Vascular leakage was classified as 'fast' or 'slow' based on the time it took for extravascular fluorescence intensity to plateau. 'Fast' responses rose within the first 2 min following exposure and plateaued within 10 min. 'Slow' leakage was characterized by reaching peak extravascular fluorescence after longer than 10 min, sometimes with a delay in BBB leakage onset following FUS+MB treatment.
Cell activity data analysis
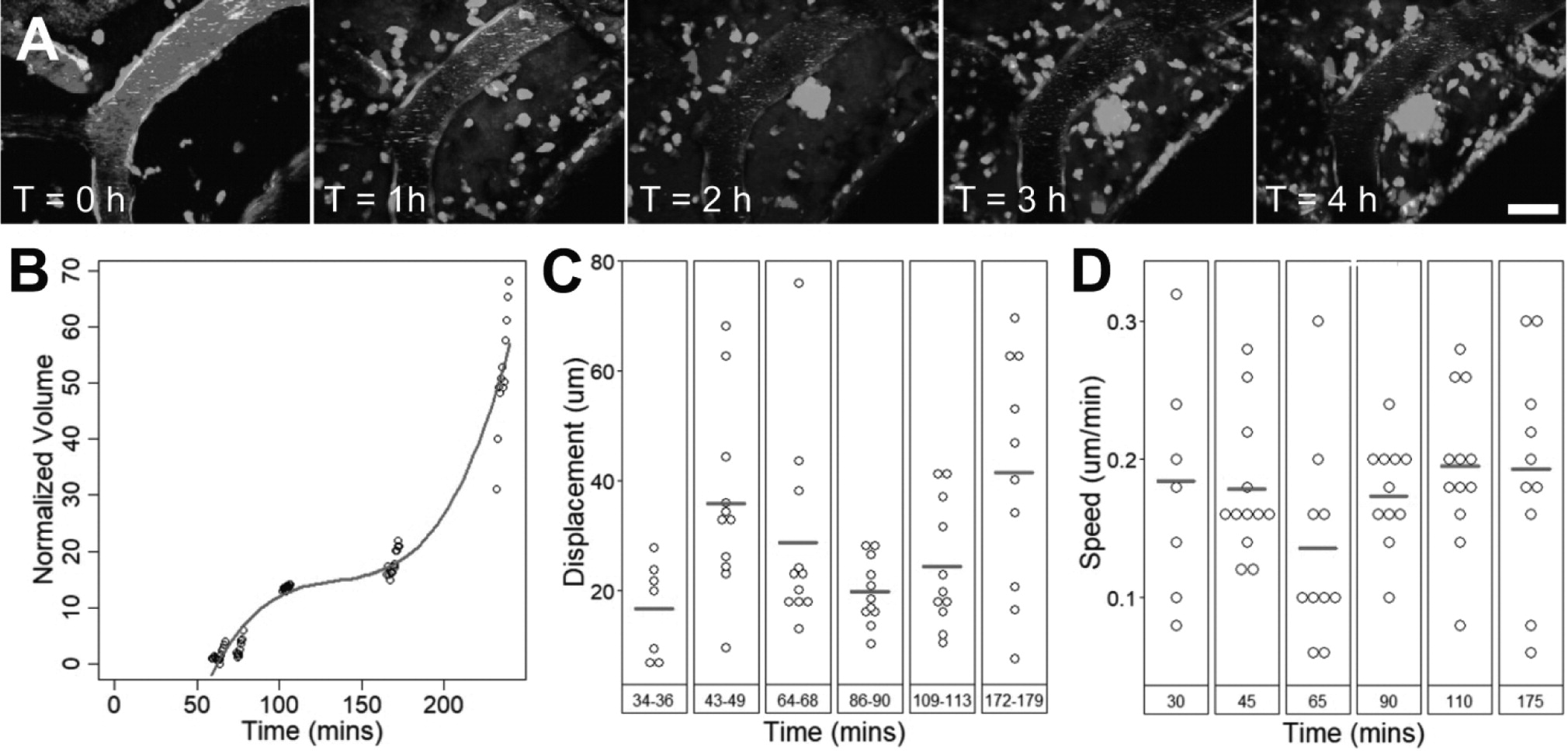
Two-photon fluorescence microscopy image stacks were analyzed with Imaris (Bitplane, Belfast, UK). 3D reconstructions of blood vessels were created using the semi-automated Surface module. In images with high background noise, such as post-treatment images in which the intensity of dextran in the extravascular space was higher than that in the intravascular space, blood vessels were manually contoured. EGFP+ cells were detected using the semi-automated Surface module for XYZT images and Spots module for XYT images. Cell activity was automatically tracked using the Track module and manually corrected. For cell displacement measurements, a minimum of ten cells were tracked. Displacement was calculated as the distance between a tracked cells' first and last position.
To evaluate the proportion of blood vessels covered by fluorescent cells, the volume or area of blood vessels and cells were measured by creating a Surface in their respective channels.
Identification and Quantification of Participating Peripheral Immune Cells
Immunohistochemistry and confocal microscopy
Immediately after imaging, rats were injected with a ketamine-xylazine cocktail and transcardially perfused with ice-cold saline and freshly prepared 4% PFA in phosphate buffer (0.1 M, pH 7.2). Brains were extracted and kept in 4% PFA overnight at 4°C, transferred to 30% sucrose at 4°C until saturated, and embedded in OCT using a dry ice and ethanol slurry. Brain tissue was cut coronally into 40 μm sections using a cryostat and separated into 6 series. Brain tissue from 9 sonicated animals and 2 control animals were analysed using immunohistochemistry; 6-10 sections were analysed per animal. Sections were stored in cryoprotectant at -20°C.
Prior to staining, brain sections were washed to remove cryoprotectant (wash buffer: 0.1% Triton X-100 in PBS; 10 min x 3 washes). For antigen retrieval, sections were treated with Rodent Decloaker (Biocare Medical; diluted 1:10 in ddH2O) at 70-80°C for 7 mins, and immersed in an ice bath for 1 h [
27]. Sections were washed, incubated in blocking solution for 1.5 h at room temperature (blocking solution: 0.1% Triton X-100, 5% donkey serum, 1% BSA), in primary antibodies for 48 h at 4°C, washed, incubated in secondary antibodies for 2 h at room temperature, and washed. Sections were mounted using Fluoromount-G (Invitrogen, USA).
The following anti-rat primary antibodies were used:to stain blood vessels, mouse anti-rat endothelial cell antibody 1 (RECA1; MCA970R; BioRad, Irvine, USA), mouse anti-PECAM-1 (also known as CD31, ab24590), and mouse anti-glucose transporter 1 (GLUT1, ab40084); to stain astrocytes, chicken anti-GFAP (ab4674); to stain neutrophils, rabbit anti-myeloperoxidase (MPO, ab9535). The following secondary antibodies were used: goat anti-chicken Alexa Fluor 488 (ab150169, abcam; Cambridge, USA), donkey anti-rabbit Alexa Fluor 568 (ab175692), donkey anti-mouse Alexa Fluor 647 (ab150107), goat anti-chicken Alexa Fluor 647 (ab150171). Primary antibodies for MPO and RECA-1 were optimized in spleen and liver tissue sections as positive controls. All antibody company codes that begin with 'ab' were from Abcam (Cambridge, USA).
Immunofluorescence was detected using a Nikon A1 laser scanning confocal microscope (Tokyo, Japan) or Zeiss Cell Observer spinning disk confocal microscope (Oberkochen, Germany), using a 20X air or 63X oil-immersion objective lens. Z-stacks were acquired using a step-size of 2 μm or 1 μm, respectively. Images were compiled using the Bio-Formats plugin in FIJI (Fiji Is Just ImageJ [
28]).
Image processing of confocal images
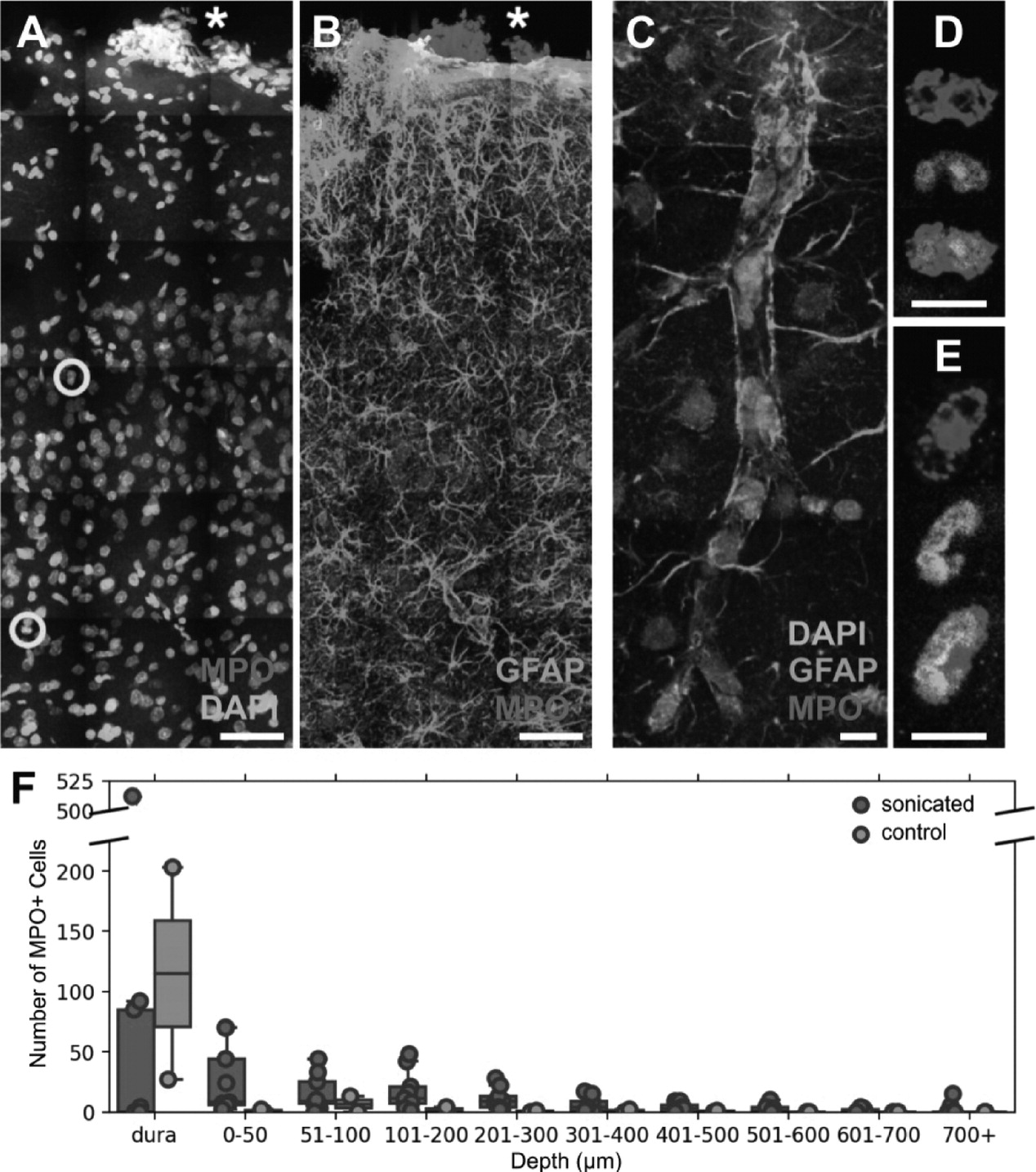
Two factors were used to identify polymorphonuclear neutrophils: positive staining for MPO, and multi-lobed nuclei. This excluded eosinophils, which have multilobed nuclei but do not express MPO [
29], and circulating monocytes, which may weakly express MPO prior to becoming resident tissue macrophages [
30] but have ellipsoidal nuclei. Astrocytes may also express MPO but do not have multilobed nuclei [
31] and are easily distinguished by their morphology.
To evaluate the number of neutrophils and relevant depth in the cortex found in FUS+MB treated and control animals, means and standard deviations were evaluated across all brain sections in each animal. Means and standard errors of the mean were calculated for controls and FUS+MB treated groups.
Magnetic resonance guided FUS treatment and analysis
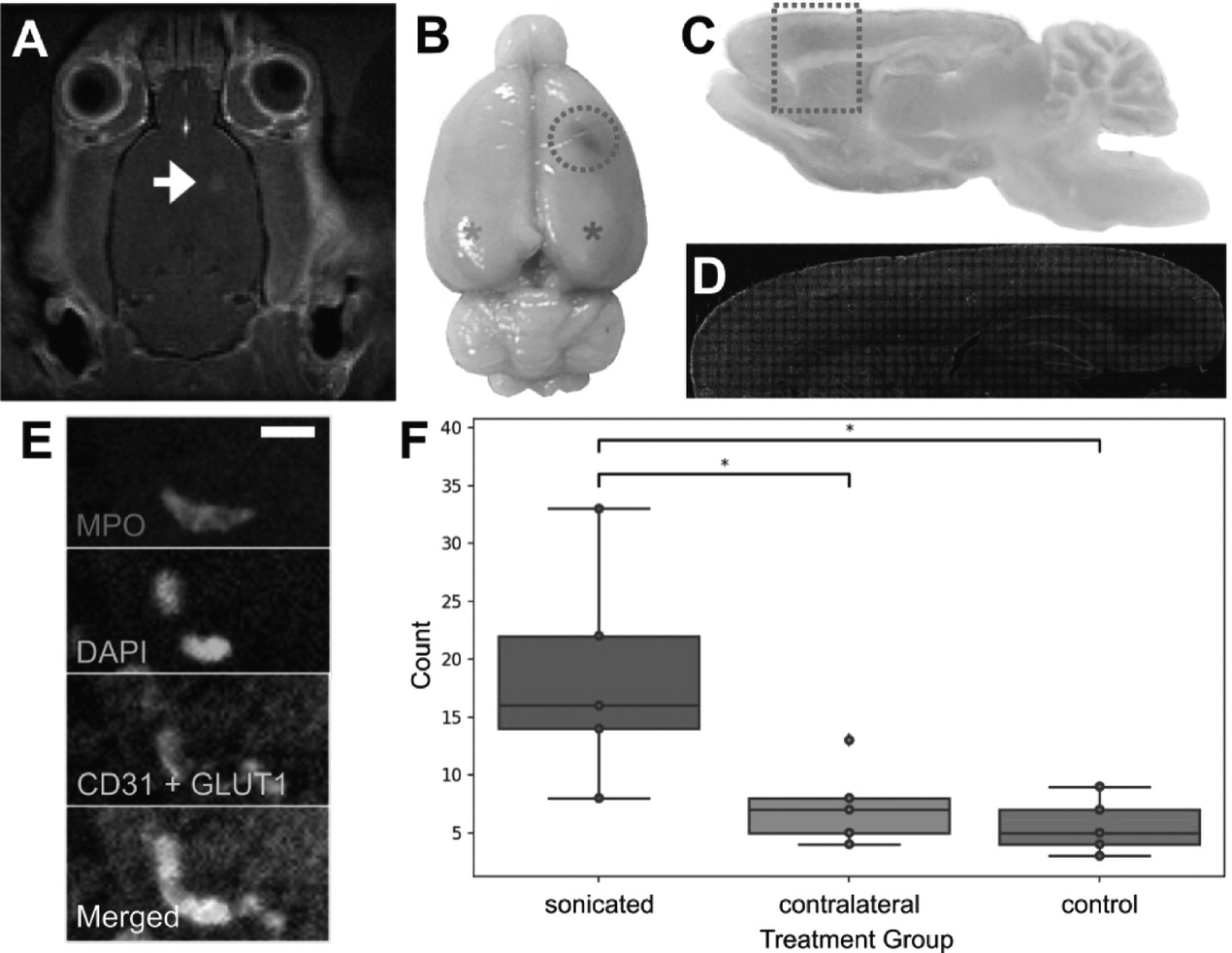
To assess the presence of neutrophils in FUS+MB treated animals without cranial windows, 5 wild-type Wistar rats (400-450 g) underwent magnetic resonance imaging guided (MRg) FUS+MB treatment, and 5 wild-type Wistar rats (400-450 g) underwent the same procedure (anesthesia, and MB and gadolinium injections) but were not exposed to FUS. Both animals were anesthetized using isoflurane. Apart from inserting a tail vein catheter and removing the fur on the top of the head, no other surgical procedures were performed.
MRgFUS experiments were performed using the LP-100 system (FUS Instruments, Toronto, Canada), consisting of a spherically curved focused transducer, an in-house manufactured PZT fiber-optic hydrophone (Precision Acoustics Ltd, UK) positioned in a 25 mm opening in the center of the transducer, and three-axis motorized positioning system. The transducer was submerged in a tank of degassed, deionized water and matched to 50 Q, 0° at its fundamental frequency by a matching circuit (F = 580 kHz, focal number = 0.8, diameter = 75 mm). FUS exposure was delivered at 10 ms bursts and 1 Hz burst repetition frequency, for a total duration of 120 s. The positioning system was coregistered with the animal's brain via T2-weighted images (TR = 2000, TE = 60) obtained using a 7T MRI scanner (BioSpin 7030, Bruker, Billerica, MA). Definity
TM MBs (diluted 1:10 v/v in saline, 0.02 ml/kg; Lantheus Medical Imaging, MA) were injected into the tail vein catheter immediately before the start of FUS exposure. One target point was chosen in the motor cortex. MRgFUS experiments were conducted using an acoustic controller to enhance BBB permeability without causing tissue damage. Specifically, acoustic pressures were increased at 8 kPa increments until the magnitude of subharmonic emissions exceeded the mean of the baseline plus 10 standard deviations of the mean, at which point pressure was automatically reduced by 50% for the remainder of the sonication duration [
32]. Following sonication, gadolinium-based contrast agent (0.2 ml/kg; Gadovist, Scherig AG, Berlin, Germany) was administered via tail vein catheter, and contrast enhanced T1-weighted MR images were acquired to assess BBB permeability.
Animals were perfused with ice-cold saline and fresh 4% PFA within 1 hour after FUS+MB or sham treatment. Brains were harvested, processed, cut, stained, and imaged, as described above. To compare the number of neutrophils found in the FUS+MB treated hemisphere, contralateral hemisphere, and control animals, 5 brain sections were evaluated per animal. The operator was blinded to the treatment groups.
Role of FUS+MB Recruited Neutrophils in Phagocytosis of Aβ
To determine whether neutrophils recruited by FUS+MB BBB treatments assisted in the clearance of Aβ plaques, 7-month-old animals of the TgCRND8 mouse model of AD were administered 3 to 5 biweekly (1 treatment every 2 weeks) MRgFUS+MB BBB treatments, targeted to the bilateral hippocampi [
14]. MRgFUS+MB BBB treatments were conducted as previously described. The TgCRND8 mouse model is an early onset model of AD that overexpresses a double mutant form of human APP 695 (Swedish (KM670/671NL) + Indiana (V717F) mutations) [
33]. By 7 months-of-age, TgCRND8 mice exhibit prolific thioflavin S-positive and dense-core plaque deposition, cognitive impairment, activated microglia and astrocytes [
34], and synaptic loss [
35]. Four TgCRND8 animals were given biweekly FUS+MB BBB treatments, and 5 TgCRND8 animals were given sham treatment, consisting of the same preparation and anesthesia exposure but without sonication. Animals were sacrificed on the tenth week, two weeks following the fifth FUS+MB or sham treatment, at 9.5-months-old.
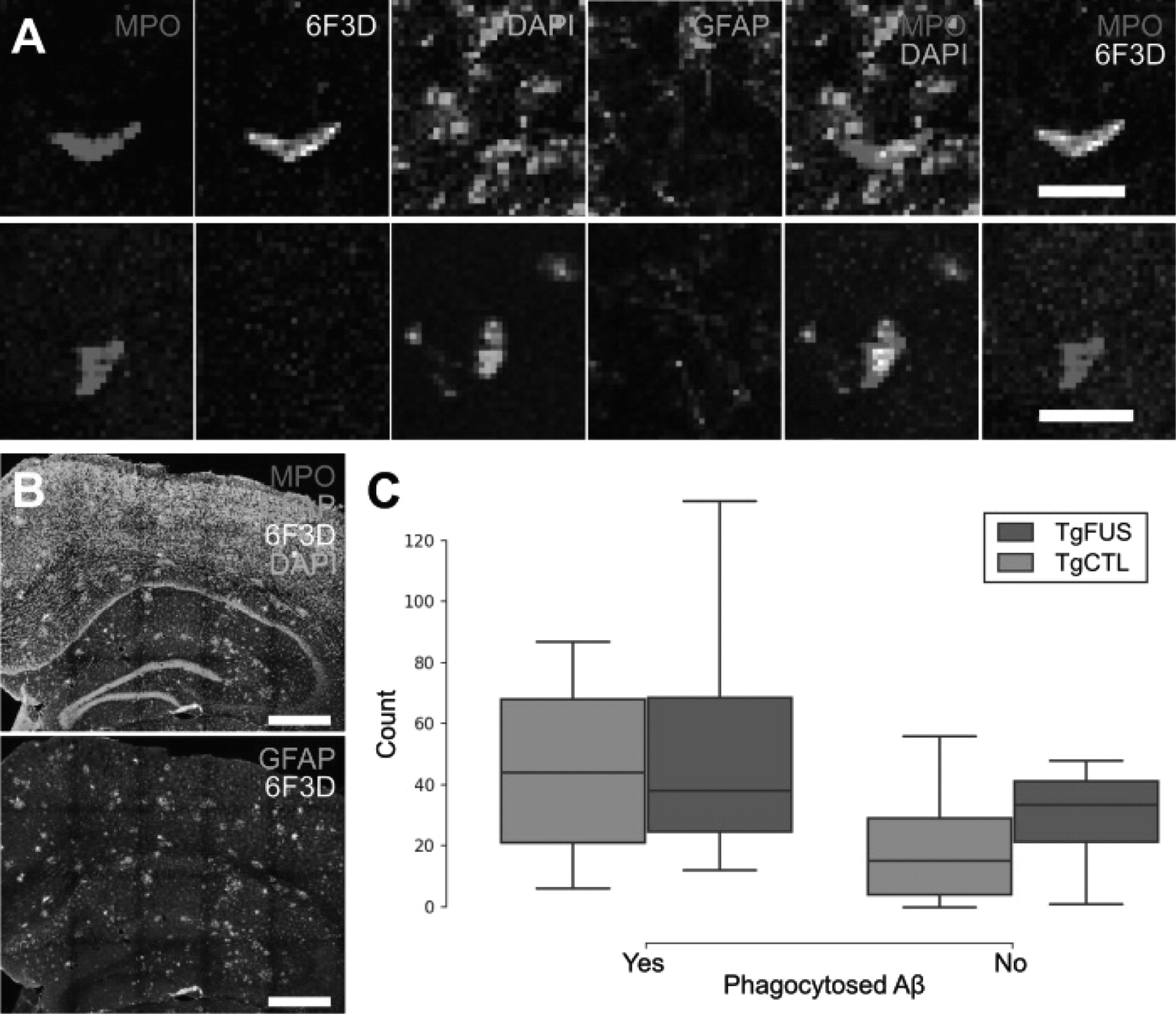
Animals were perfused and brain tissue was harvested as described above. For immunohisto-chemical analyses, the following primary antibodies were used: anti-mouse Ly6G for neutrophils (127602; BioLegend, San Diego, USA), 6F3D for Aβ plaques (Dako, Glostrup, Denmark), and chicken anti-GFAP for astrocytes (ab4674; Abcam, Cambridge, USA). Five brain sections were analyzed per animal. Stained brain sections were scanned with a confocal microscope and analyzed with FIJI.
Statistical Analyses
All statistical analyses were performed using Python.
To compare the number and depth of neutrophils in FUS+MB treated and control animals in the 2PFM experiments, the two groups were compared using a Mann-Whitney U test for unpaired non-parametric data at each depth.
In the analysis of total neutrophils counted in MRgFUS+MB BBB treated brain hemispheres ('sonicated' group, n = 5), contralateral hemispheres ('contralateral' group, n = 5), and control animals ('control', n = 5), group means were compared using a one-way ANOVA and Tukey's test of multiple comparisons. To compare peak BBB permeability time, blood vessel groups ('arteriole', 'venule', 'capillary') were compared using one-way ANOVAs. In TgCRND8 animals, neutrophil counts were compared in FUS+MB treated animals ('TgFUS', n = 4) compared to control animals ('TgCTL', n = 5) using a f-test.
Speeds of tracked intravascular cells in affected arterioles and venules were compared using a two-way ANOVA with 'time' and 'blood vessel type' as the independent groups.
Data is available upon reasonable request.
Discussion
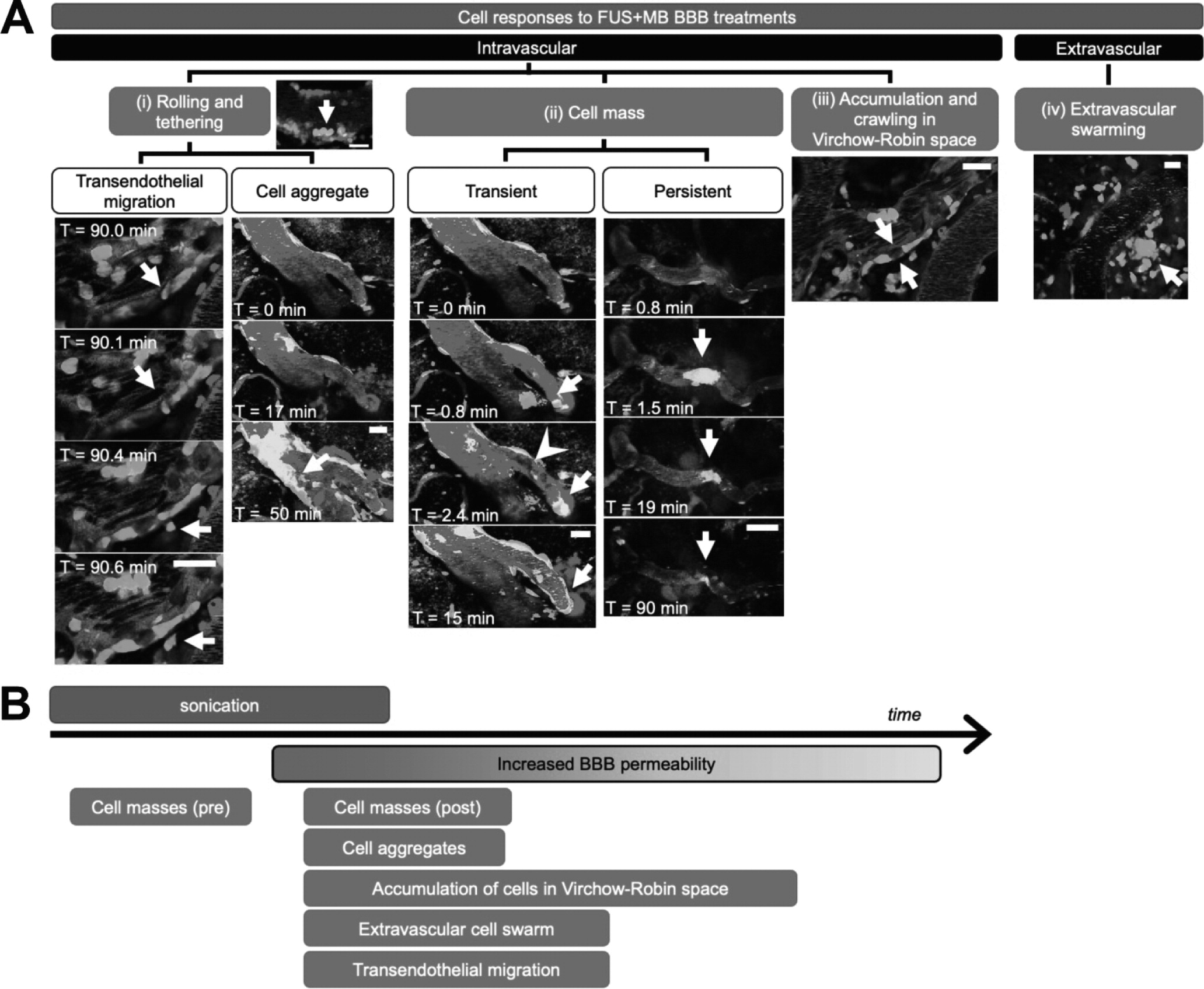
To our knowledge, this is the first report describing immune cell responses to FUS+MB mediated BBB treatments with real-time
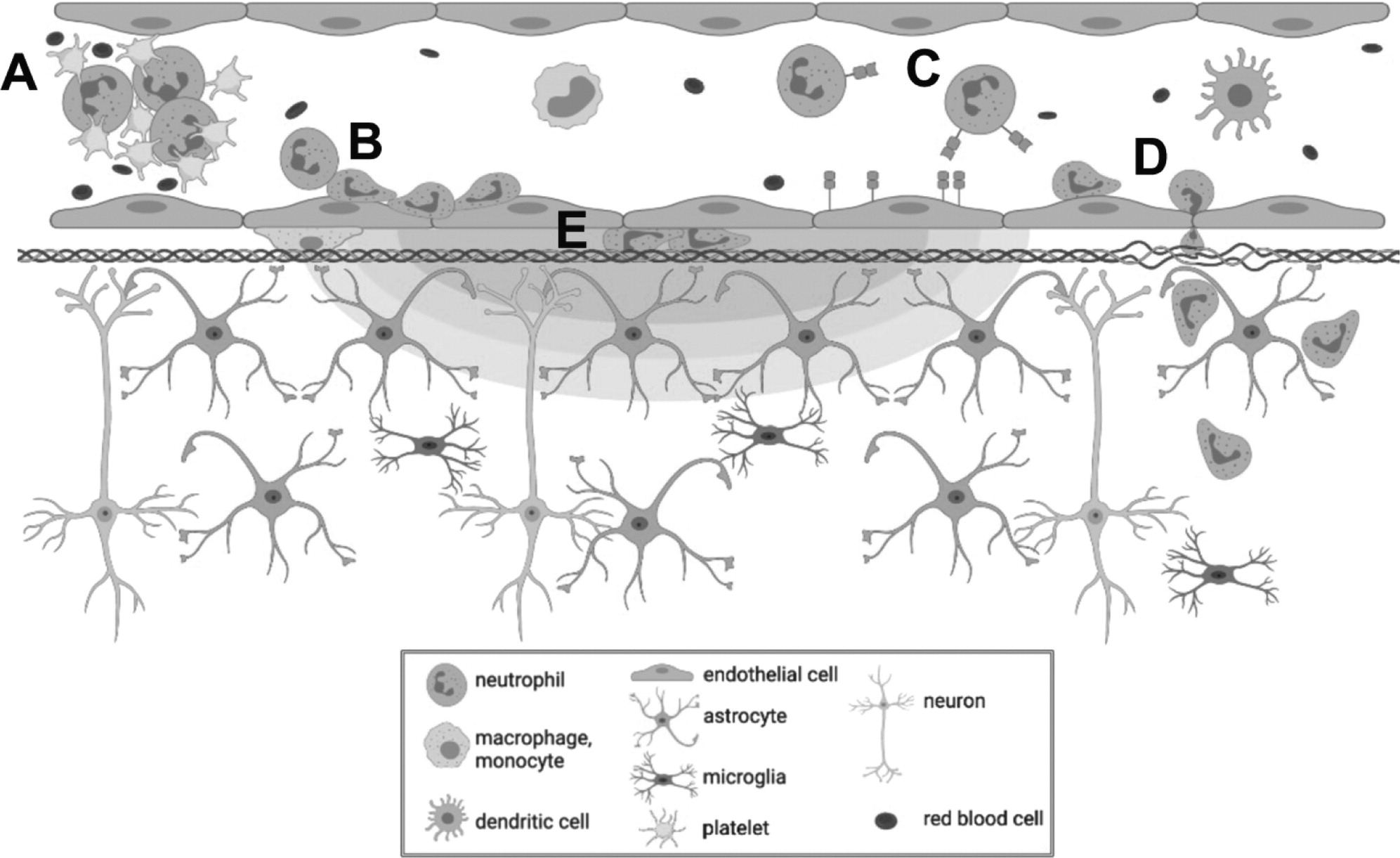
in vivo imaging. The interaction of MBs with vascular endothelium during sonication recruited compact groups of cells (preformed 'cell masses') to blood vessel segments before and after the onset of increased BBB permeability, as detected by the leakage of dextran into the extra vascular space. Preformed cell masses that spanned the diameter of the lumen ('cell occlusions') were observed to obstruct blood flow. Following the onset of BBB leakage, cells were observed to respond individually and as aggregates in the intravascular space. Individual cells tethered, rolled, and adhered to the luminal aspect of affected blood vessels, at times leading to the formation of cell aggregates or transendothelial migration. Cells were also observed to crawl and accumulate within the Virchow-Robin space of an affected venule, and formed an extravascular swarm around an affected arteriole. A schematic of the different cell responses observed is shown in
Figure 10. One of the primary cell types participating in these responses was identified to be neutrophils. The number of neutrophils found in sonicated brain hemispheres was found to be significantly higher than that found in the contralateral hemisphere, and that in control animals, indicating that FUS+MB BBB treatments induce local recruitment of neutrophils.
To determine whether neutrophils recruited to FUS+MB affected vasculature can aid in Aβ plaque clearance, 7-month-old TgCRND8 animals were administered 3 to 5 biweekly FUS+MB BBB treatments, and sacrificed at 9.5-months-of-age, 2 weeks following the fifth treatment. This treatment regime has been previously demonstrated to result in significant reduction in Aβ plaque load [
14]. While neutrophils containing Aβ plaques were found, indicating an ability to phagocytose Aβ, the number of neutrophils with and without phagocytosed Aβ did not differ significantly between FUS+MB treated and control TgCRND8 animals. While this may suggest that recruited neutrophils did not aid in plaque clearance, it is also possible that the effect size of neutrophil phagocytosis of Aβ plaques may have been washed out by the time of sacrifice. The similar number of total neutrophils in the FUS+MB treated and control TgCRND8 animals suggests that the latter may be the case, as results differ from the neutrophil counts counted in animals sacrificed 1 h following FUS+MB BBB treatments (compare
Figure 8F with
Figure 9C). Furthermore, the immediate but transient response of neutrophils is well-described in literature: neutrophils are the first line of defense in acute inflammation, and have a short lifespan in tissue (0.75 days in mice, 5.4 days in humans [
39]). In addition, extravasated neutrophils may return to circulation through reverse transendothelial migration [
40].
Despite their limited effect on Aβ clearance, FUS+MB induced recruitment of neutrophils may aid in Aβ clearance through downstream recruitment of other leukocytes. Specifically, neutrophils can recruit blood monocytes to inflammatory sites through secretion of soluble factors such as C-C chemokines [
41]. Recruited monocytes and macrophages have longer lifespans in tissue [
42] and can emigrate to the lymphatics system [
43]. As such, monocytes and macrophages may have a greater capacity than neutrophils in clearing Aβ [
44]. For example, depletion of monocytes resulted in worsened outcomes following parasite infection, whereas depletion of neutrophils did not result in any pathological changes, suggesting that monocytes, but not neutrophils, may play a protective role [
45].
The cell responses observed in this study, such as transendothelial migration of leukocytes and leukocyte aggregate adhesion, are known components of acute inflammation [
46]. Transcriptional changes indicative of an acute inflammatory response following FUS+MB BBB treatments have been previously reported, including an upregulation of genes that support immune cell migration
(Sele, Cxcll, Ccl7) [
19,
20,
47]. This study provides evidence that the acute inflammatory response, observed as leukocyte recruitment, begins during FUS exposure, whereas the earliest time point measured for previous studies were 5 mins [
48], and 6 h following FUS+MB treatments [
19]. The speed of cell responses led to the hypothesis that neutrophils are a key participating leukocyte, as they are the most abundant leukocytes in circulation, as well as the first immune cells to transmigrate to areas of inflammation through endothelial selectin-dependent tethering and rolling [
49]. However, the heterogeneity of cell responses observed suggests that FUS+MB BBB treatments may induce a multistep inflammatory response, involving several types of leukocytes that lead to increases in vascular permeability and recruitment [
46,
50]. Other phagocytic leukocytes with longer lifespans in tissue than neutrophils may also be aiding in FUS+MB induced Aβ clearance. Different cell types may also respond differently according to the extent of enhanced BBB permeability achieved.
The cell aggregates observed in this study may be comprised of platelets and leukocytes. In addition to myeloid leukocytes, platelets are also capable of rolling, adhering, and aggregating on activated endothelium [
51]. As part of the innate immune response, platelets can form aggregates with neutrophils and circulating monocytes, and promote neutrophil tethering to activate ECs through expression of selectins and inflammatory cytokines and chemokines [
52]. Platelets can also interact with dendritic cells, which can lead to recruitment of T cells [
50]. Recruitment of leukocytes and leukocyte-platelet aggregates may also contribute to increased microvascular permeability [
46].
In one case, an extravascular swarm of leukocytes was observed following BBB leakage. The activity and morphology of these cells resembled the rapid chemotaxis of neutrophils to sites of sterile inflammation [
53], suggesting that FUS+MB treatment in this case may have caused vascular damage. Similarly, macrophage infiltration into the brain have been observed following FUS+MB treatments using parameters designed to cause tissue damage, but not if sonication parameters and MB dosages for reversible BBB treatments that do not produce overt tissue damage were used [
18,
20,
54].
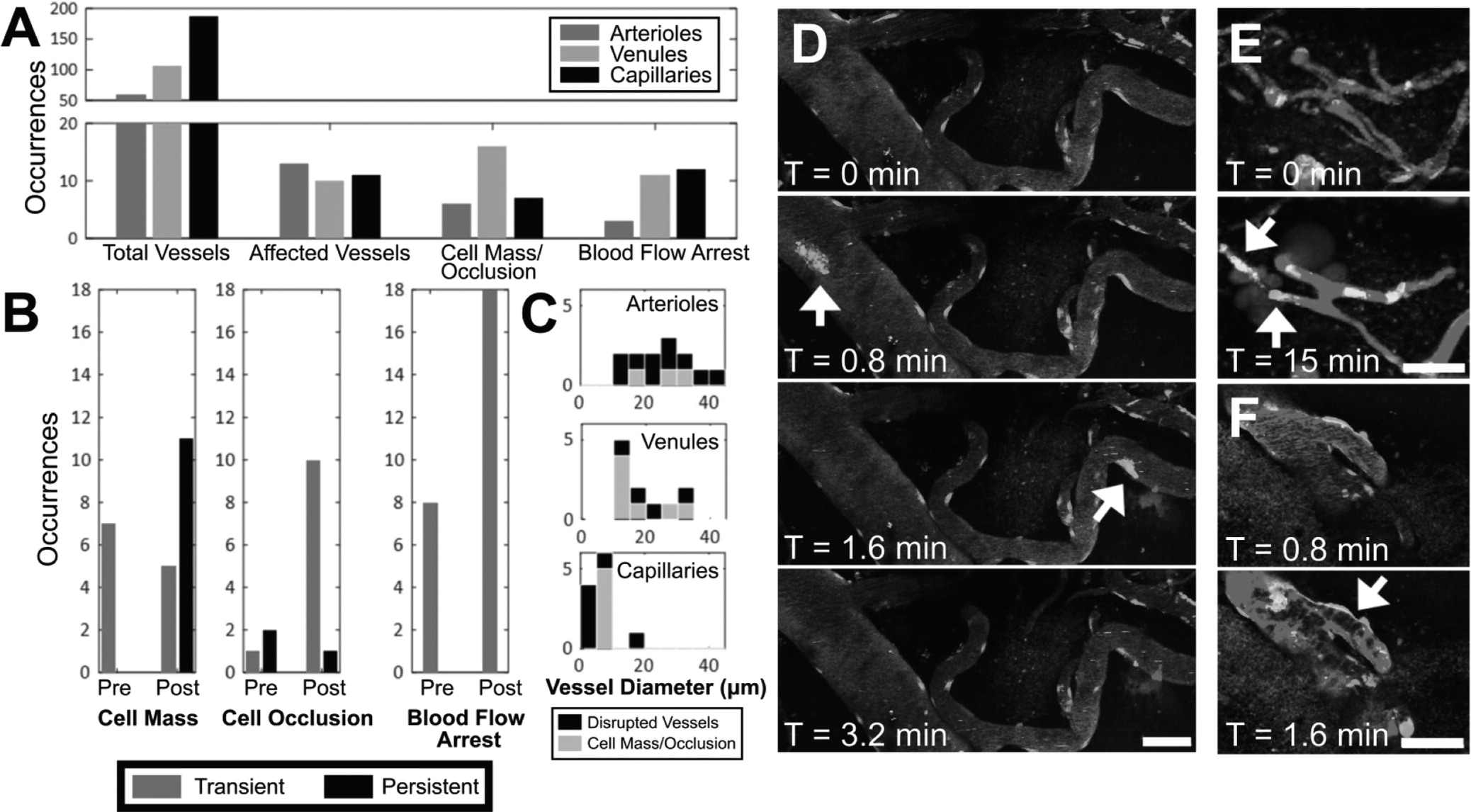
Alterations in blood flow were observed during and after FUS+MB BBB treatments, at times accompanied by cell occlusions. Reversible local disturbances in blood flow during sonication are likely due to radiation force of the propagating ultrasound wave. Similar phenomena, described as 'blood cell stasis' or 'parathrombosis', have been previously observed in human blood
in vitro [
55], and chick embryos
in vivo [
56,
57]. Persistent stalls in capillaries may affect short-term memory function [
58], and should be further examined in the context of FUS+MB treatments.
An anisotropic distribution of dextran diffusion was observed surrounding FUS+MB affected blood vessel segments. Increases in fractional anisotropy, a measure of diffusion anisotropy, have been observed in grey matter areas with immunoglobulin leakage in a rodent model of acute reversible encephalopathy [
58]. It is possible that the anisotropy observed on the microscopic scale in these FUS+MB BBB 2PFM experiments may be detectable in fractional anisotropy measurements taken from diffusion tensor imaging MRI scans, as immunoglobulin extravasation has been observed following FUS+MB BBB experiments as well [
11]. Such measurements would enable more global analyses of leakage kinetics following FUS+MB BBB treatments. The observed anisotropic distribution and constraint of dextran around blood vessels may be due to heterogeneities in the vascular wall and suggests the involvement of the extracellular matrix and/or basement membrane. For example, chronic hypertension, which leads to an increase in extracellular matrix proteins, including collagen [
60], is the main vascular risk factor for small vessel disease, which is associated with reduced fractional anisotropy [
61-
63]. Furthermore, remodelling of the extracellular matrix can be achieved by immune cells: neutrophils are a source of matrix metalloproteinases [
64], which can degrade the extracellular matrix, and have been correlated with reduced type IV collagen expression following stroke [
65]. Analysis of extracellular matrix proteins following FUS+MB BBB treatments may reveal insight into leakage kinetics and the extent of immune cell infiltration.
This study may also offer insight into purported clearance mechanisms observed after FUS+MB BBB treatments. The intravascular accumulation of cells within the Virchow-Robin space of an affected venule may also support the hypothesis that FUS+MB BBB treatments affect the glymphatic clearance pathway [
66]. In addition, the marginal increase in the number of neutrophils in the contralateral hemisphere compared to control animals suggests that FUS+MB BBB treatments result in neutrophil recruitment in a wider area than observed changes in vascular permeability, or that recruited neutrophils have a high capacity for migration. Further investigations into the cell types recruited by FUS+MB BBB treatments and the spatial and temporal extents of treatment effects are recommended.
This study provided evidence that neutrophils respond to FUS+MB BBB treatments immediately after the onset of FUS exposure. Immune responses to FUS+MB BBB treatments were observed sooner than previously reported, and may be triggered from interactions of MB oscillations with vascular walls before increases in BBB permeability were observed. While the short lifespan of neutrophils in tissue and evidence of their return to circulation suggests that their effects following FUS+MB BBB treatments may be transient, their ability to recruit other leukocytes with longer lifespans in tissue, such as monocytes, may lead to the downstream Aβ clearance observed in previous FUS+MB AD studies. This study adds to the body of literature that points to the necessity of controlling FUS+MB treatments through optimal parameter selection and real-time monitoring, and reveals aspects of the peripheral immune system that should be explored when considering future studies involving FUS+MB BBB treatments in neuroinflammatory diseases or in conjunction with drug delivery.