Cognitive Dysfunction in Major Depressive Disorder: Assessment, Impact, and Management
Abstract
Definition
| Domain | Description | Representative Tests |
|---|---|---|
| Attention | Encompasses ability to focus, maintain, and shift attention | Continuous Performance Test–Identical Pairs |
| Processing speed | Ability to rapidly perceive or respond to stimuli | Trail Making Test Part A; Digit Symbol Substitution Test; Category Fluency: Animal Naming and Phonetic Fluency |
| Working memory | Temporary storage and manipulation of information | Spatial Span subtest (WMS-III); Letter-number sequencing (WMS-III); Digit Span Forward and Backward (WAIS-R); Spatial Working Memory (CANTAB); n-Back Test |
| Verbal learning and memory | Encompasses learning, encoding, retention, and retrieval of verbal information | California Verbal Learning Test–Revised; Hopkins Verbal Learning Test–Revised; Rey Auditory Verbal Learning Test |
| Visual learning and memory | Ability to retain and retrieve visual images | Brief Visuospatial Memory Test–Revised; Rey-Osterrieth Complex Figure Test; Visual Memory Index; Pattern Recognition Memory (CANTAB); Spatial Recognition Memory (CANTAB); Delayed Matching to Sample (CANTAB); Paired Associates Learning (CANTAB); |
| Executive functioning | Ability to plan, problem solve, and inhibit affectively charged or inappropriate responses | Wisconsin Card Sorting Test; Trail-Making Test Part B; Stroop Color-Word Interference Test; Tower of London (CANTAB); Controlled Oral Word Association Test; Stockings of Cambridge (CANTAB); Intra/Extradimensional Shift Test (CANTAB) |
Epidemiology and Natural History
Prevalence of Cognitive Dysfunction in Depression
Impact of Cognitive Functioning on Clinical and Functional Outcomes
Course of Cognitive Dysfunction in Depression
Moderators of Cognitive Dysfunction in Depression
Biopsychosocial Underpinnings
Neurobiological
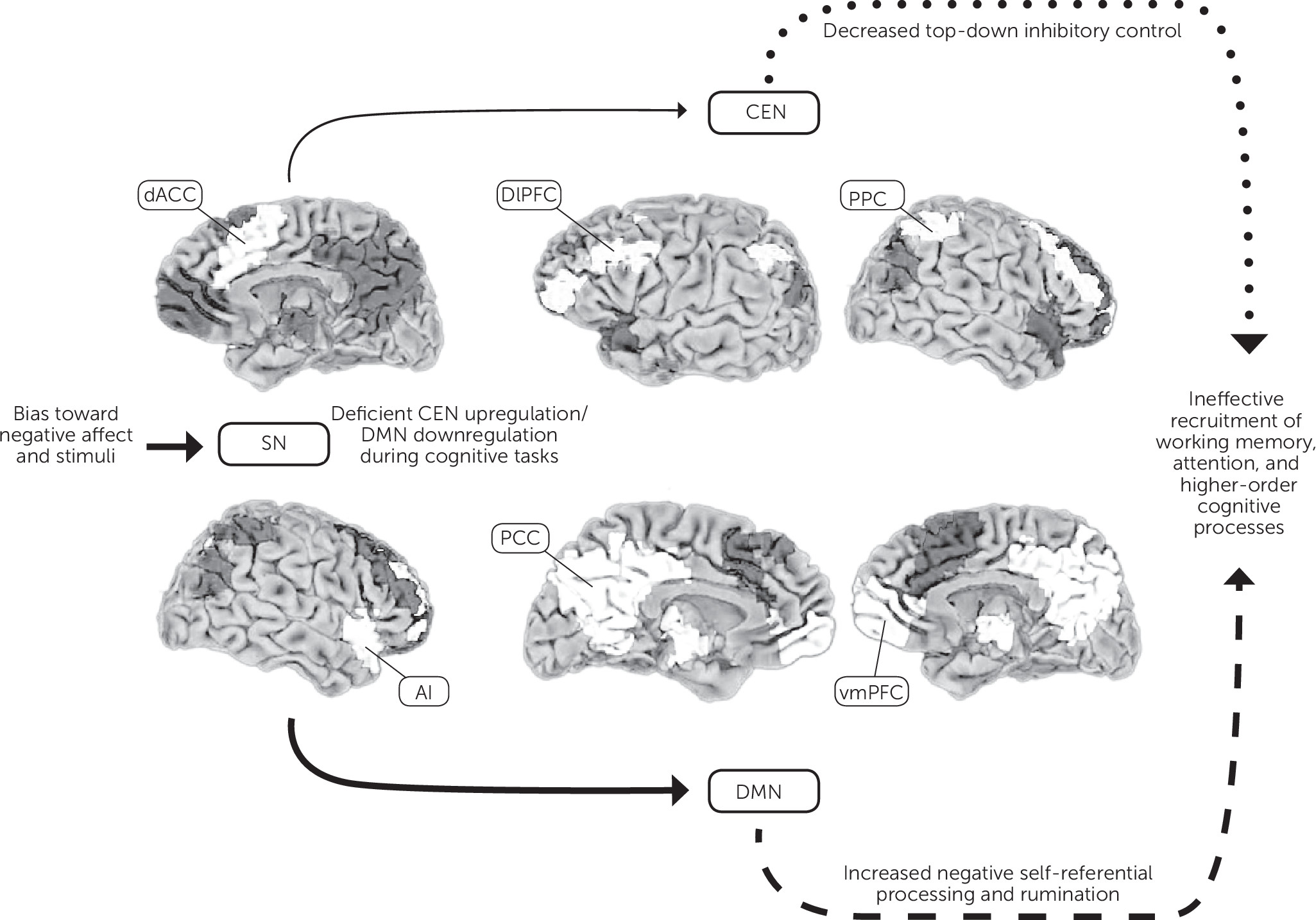
Stress and the Hypothalamic-Pituitary-Adrenal Axis
Monoamine Modulation
Cognitive Schemas and Negative Affective Bias
Assessment and Differential Diagnosis
| Study | Scale | No. of Items | Validation Sample |
|---|---|---|---|
| Iverson and Lam (108) | British Columbia Cognitive Complaints Inventory | 6 | Major depressive disorder, N=62; healthy, N=112 |
| Fava et al. (109) | Cognitive and Physical Functioning Questionnaire | 7 | Major depressive disorder, N=150 and N=50; generalized anxiety disorder, N=381 |
| Lam et al. (110) | Perceived Deficits Questionnaire–Depression | 20 (also 5-item version) | Major depressive disorder, N=400; healthy, N=400 |
Treatment and Outcomes
Antidepressant Monotherapy
| Medication | Studies | Summary of Evidenceb | Description of Studies |
|---|---|---|---|
| SSRI | |||
| Paroxetine | Ferguson et al. (114), Gorlyn et al. (115), Deuschle et al. (116), Nickel et al. (117) | 0*/+/+/+ | DBRCT (N=23, 20–40 mg/day); did not separate from placebo. Three trials found improvement compared with baseline (Ns=30, 24, and 44) |
| Escitalopram | Herrera-Guzmán et al. (118, 119), Soczynska et al. (120) | +/+ | Improvements in objective cognition compared with baseline performance and untreated controls (Ns=36 and 19). |
| Sertraline | Constant et al. (121) | + | Improvements in attention, executive function, and psychomotor speed (N=20) |
| Fluoxetine | Chang et al. (122), Richardson et al. (123), Levkovitz et al. (124) | +/+/+ | Three trials showing improvement in objective cognition compared with baseline (Ns=73, 18, and 8) |
| SNRI | |||
| Duloxetine | Herrera-Guzmán et al. (118, 119), Mahableshwarkar et al. (125) | 0*/+ | Improved memory, attention, and executive function compared with baseline in one sample (N=37). DBRCT (N=176, 60 mg/day) showed improved subjective cognition but no difference in processing speed and functional capacity compared with placebo. |
| Venlafaxine | Chang et al. (122) | + | Improved attention and executive function compared with baseline (N=72) |
| SSRI/SNRI | Nagane et al. (126) | − | Remitted patients receiving unspecified SSRI or SNRI antidepressants showed decreased visual memory compared with nonmedicated controls (N=21) |
| NDRI: buproprion | Gorlyn et al. (115), Soczynska et al. (120), Herrera-Guzmán et al. (127) | +/+/+ | Improved verbal and nonverbal memory compared with baseline in two studies (Ns=17 and 27) and improved visual memory and processing speed (N=20) |
| NaSSA: mirtazapine | Borkowska et al. (128) | + | Improvements in working memory, processing speed, and executive function after six months of treatment compared with baseline (N=71) |
| NRI: reboxetine | Ferguson et al. (114) | +* | DBRCT (N=25, 8–10 mg/day) demonstrated improved attention and processing speed compared with placebo |
| Multimodal vortioxetine | Mahableshwarkar et al. (125), McIntyre et al. (129) | +*/+* | DBRCT (N=168, 10–20 mg/day) showed improved processing speed and functional capacity compared with placebo; DBRCT of 10 mg/day (N=195) and 20 mg/day (N=207) found improvements in subjective and objective cognition compared with placebo at both doses |
Adjunctive Pharmacotherapy
Neuromodulation
Psychotherapy
Conclusions and Future Directions
References
Information & Authors
Information
Published In
History
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

