There is increasing evidence that immune parameters are altered in different psychiatric patient populations, including patients with major depressive disorder (MDD). Such alterations in patients with MDD include functional changes of cellular immune parameters, such as neutrophil and monocyte phagocytosis and cell number of neutrophils and lymphocytes,
1 as well as changes in the concentrations of different cytokines and cytokine-receptors.
1–3 Among these, interleukin-6 (IL-6) has been examined under several experimental conditions in depressed patients, but results are conflicting. Whereas several studies reported increased concentrations of IL-6 in depressed patients,
4–8 others did not find different levels in depressed patients and control subjects
9 or found decreased levels in stimulated peripheral blood mononuclear cells.
10–12 A recent meta-analysis
13 that reviewed all relevant articles published before 2008, found a positive association between levels of IL-1, IL-6, and depression. Inconsistent results were also found for serum concentrations of the soluble receptor for IL-2 (sIL-2R): Some studies reported increased levels
4,5,9,14 whereas another study found decreased levels,
15 and two others reported no change as compared with normal controls.
8,15 Two studies investigated serum levels of the IL-1 receptor antagonist (IL-1RA) in depressed patients: One study found increased levels,
7 whereas another study found no alteration.
11 A recent study
16 found increased IL-1RA levels in a large sample of depressed patients, as compared with healthy controls, and attributed this to an endogenous protection process against depression.
Method
Subjects
A group of 19 drug-free patients with MDD according to the Structured Clinical Interview for DSM-IV were recruited for the study; 4 patients dropped out, 1 because of problems with the blood-drawing procedure and 2 because of exclusion criteria that had not been recognized at the time of inclusion. One patient withdrew her consent during the study, before the TSD night. Another patient had a mood deterioration on the morning after the TSD night, and therefore this patient was prematurely retired from the study. Her available cytokine data were included in the analysis. The 16 patients (10 women, 6 men) had a mean age of 34 (SD: 13) years and a mean body mass index (BMI) of 22.6 (SD: 3.8) kg/m2. Among the patients, 40% (6 of 16 patients) were smokers (daily cigarettes >10, except in 1 patient). A group of 17 healthy subjects with no personal or family history of psychiatric disorders was recruited. One subject dropped out because of problems with the blood-drawing procedure. The remaining subjects (11 women, 5 men) had a mean age of 27 (SD: 8) years and a mean body mass index of 22.4 (SD: 1.7) kg/m2; 4 out of 16 subjects were smokers, all of them moderate (<10 cigarettes per day). There were no differences between the groups regarding age, sex, or BMI. The smoker/nonsmoker ratio did not differ significantly between patients and controls. Controls were screened for depressive disorder with the Beck Depression Inventory (BDI) and Hamilton Rating Scale for Depression (Ham-D) questionnaires, but showed no clinical levels. The mean Pittsburgh Sleep Quality Index (PSQI) was 11.9 (SD: 4) for depressed patients and 3.3 (SD: 1.3) in healthy controls (p=0.002). During the study, smoking was not allowed. Exclusion criteria for participation were significant medical disorders or infectious diseases, which were ruled out by a careful physical examination, ECG, EEG, and routine laboratory parameters, including a drug screening test. Additional exclusion criteria were a history of alcohol or drug abuse and any intake of psychotropic medication or anti-inflammatory drugs within 7 days before the investigation, except contraceptives in the group of depressed patients. The drug-free interval had been longer than 4 weeks in all but 1 patient, who stopped doxepine intake 7 days before the study. Restless legs syndrome and sleep apnea syndrome were ruled out by interview and by polysomnography in all subjects and patients. The study was performed according to the Declaration of Helsinki, and the study protocol was approved by the ethical committee of the University of Freiburg. Informed consent was obtained from all subjects and patients before their participation in the study.
Sleep Deprivation Protocol
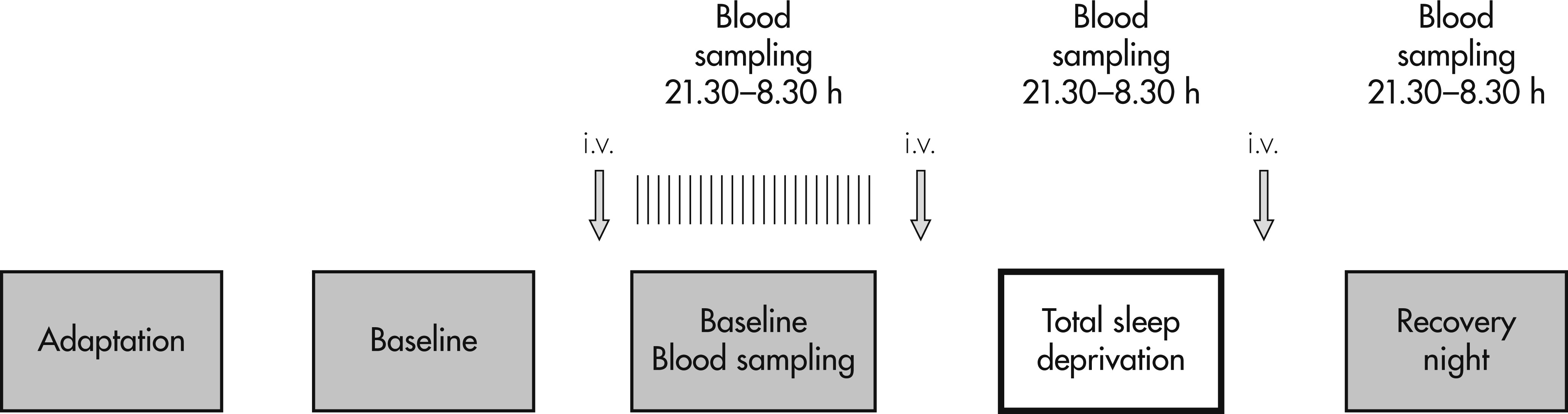
All subjects spent 5 nights in our sleep laboratory (
Figure 1). Nights 1 and 2 served for adaptation to the laboratory conditions. During Nights 3, 4, and 5, blood was sampled every half-hour, beginning at 9:30 P.M., until 8:30 A.M. the next day. Blood samples were obtained through an in-dwelling catheter, which, was placed in a forearm vein 3 hours before the beginning of blood sampling every evening and perfused by isotonic saline at a low speed (25 ml/hr). Blood samples were taken from an adjoining room through a long polyethylene tubing (total amount per night ∼110 ml). At the end of blood sampling in the morning after Nights 3, 4, and 5, the i.v. catheter was removed. In the evening, a new catheter was inserted, in order to avoid local inflammation and increase of cytokines due to the catheter.
A total sleep deprivation (TSD) was performed during Night 4; that is, subjects and patients stayed awake from 7:00 A.M. the morning after Night 3, until 11:00 P.M. the next evening. From the evening of Day 3 until the morning after the recovery night, subjects stayed in the research ward. During the 40 hours of TSD, subjects were strictly advised not to sleep, and, therefore, they were constantly observed by a team of three research fellows. No major physical activities were allowed. Subjects and patients were allowed to remain in a semi-supine position in bed or to sit in a comfortable armchair and were allowed to read or watch TV.
Polysomnographic Assessments
Sleep was recorded by standardized polysomnography for 2 nights before the beginning of the neuroendocrine protocol for adaptation to the sleep laboratory (Night 1) and to get baseline sleep parameters (Night 2), as well as during the catheter Nights 3 and 5 (for the method of polysomnography in our sleep laboratory see Voderholzer et al.
28,29).
Psychometric Measurements
Before inclusion into the study, participants completed a 21-item Hamilton Rating Scale for Depression (Ham-D). During Study Days 3, 4, 5, and 6, that is, before and after the night with TSD, a modified Ham-D, with 6 items (Ham-D-6
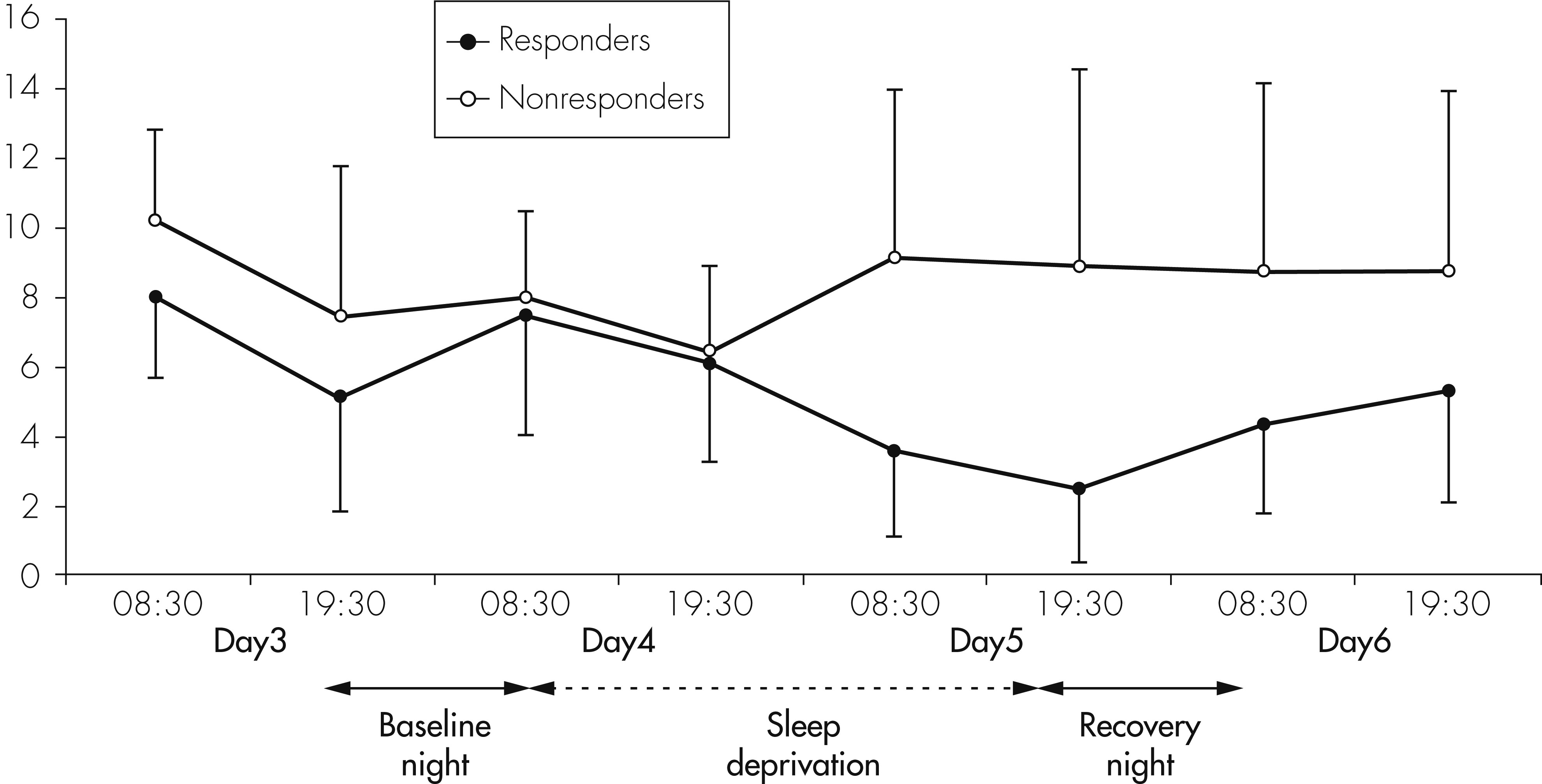
30), was filled out twice daily, in the morning and in the evening. Responder status to TSD was defined as a ≥30% reduction of the mean values of morning and evening Ham-D-6 scores after the night of TSD.
Determination of IL-6, IL-1RA, IL-2R, and Cortisol Concentrations
We have chosen to determine IL-6, IL-1RA, IL-2R, and cortisol because of the various studies reporting alteration of those cytokines in depressed patients. IL-1RA and IL-2R were used instead of the respective ligands IL-1 and IL-2, since those cytokines are only occurring in low levels in plasma or serum, approaching the detection level of our ELISA, and thus not providing reliable results. IL-6 was directly measured every 2 hours between 10:00 P.M. and 8:00 A.M. by an ultrasensitive ELISA (GE Healthcare) with a lower detection limit of 0.1 pg/ml. The intra-assay variability is 6.1%; the interassay variability, 19.6%. IL-1RA was determined with an immunoassay by R&D Systems in three samples per night (first sample period: 10:30 P.M.–1:00 A.M.; second sample period: 1:30 A.M. –4:00 A.M.; third sample period: 4:30 A.M. –7:00 A.M., respectively). The lower detection limit is 20 pg/ml; the intra-assay variability ranges between 3.1% and 6.2%; the interassay variability, between 5.0% and 6.7%. Concentrations of sIL-2R were measured by a high-sensitivity ELISA (Immunotech), with a lower detection limit of 5 pMol, in three samples per night, as for IL-1RA. The intra-assay variability ranged between 4.9% and 6.6%; the interassay variability, between 10.6% and 15.1%. Cortisol levels were measured with an immunometric assay (Nichols Institute Diagnostics). All measurements were made in duplicate. Because of the comparatively high interassay variability, all samples of one subject or patient were measured using one ELISA plate.
Statistical Analysis
Results are expressed as mean (standard deviation [SD]). Nocturnal concentrations of IL-6, IL-1RA, and sIL-2R were analyzed by calculating the mean values of all samples of each night and then analyzed using linear mixed-effects analysis with the linear model GROUP*NIGHT. Random effects were modeled as one constant term per subject (repeated-measures design); p values ≤0.05 were considered to be significant. In the case of a significant effect by ANOVA, confirmatory t-tests with Bonferroni adjustment for multiple comparisons were calculated. To evaluate the impact of the clinical response to TSD in depressed patients, the impact of TSD on cytokines was evaluated in Responders and Nonresponders to TSD. For response analysis, repeated-measures 3×3 ANOVA, with the factors Time (baseline, sleep deprivation, recovery night) and Response (responder, nonresponder, healthy control) was calculated.
Discussion
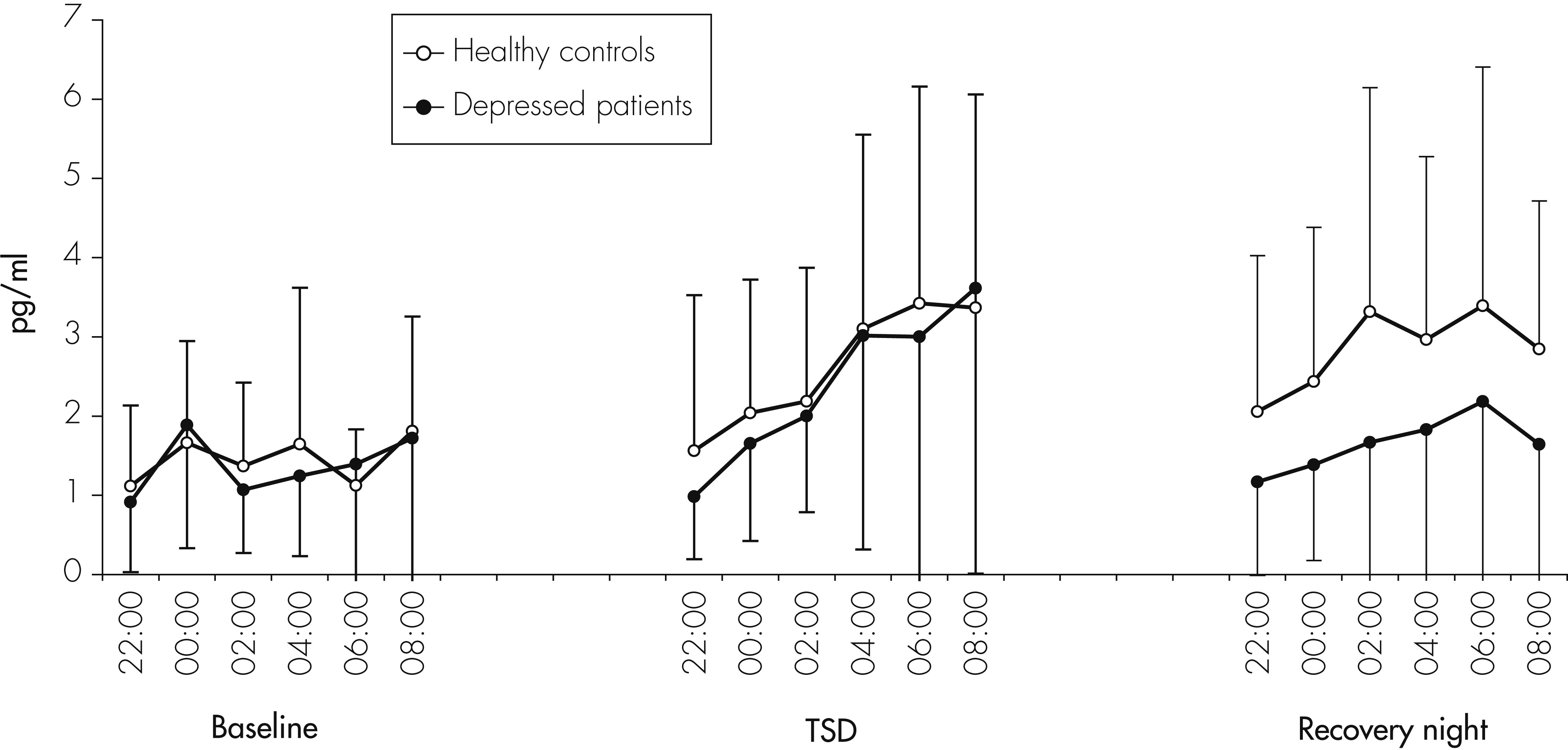
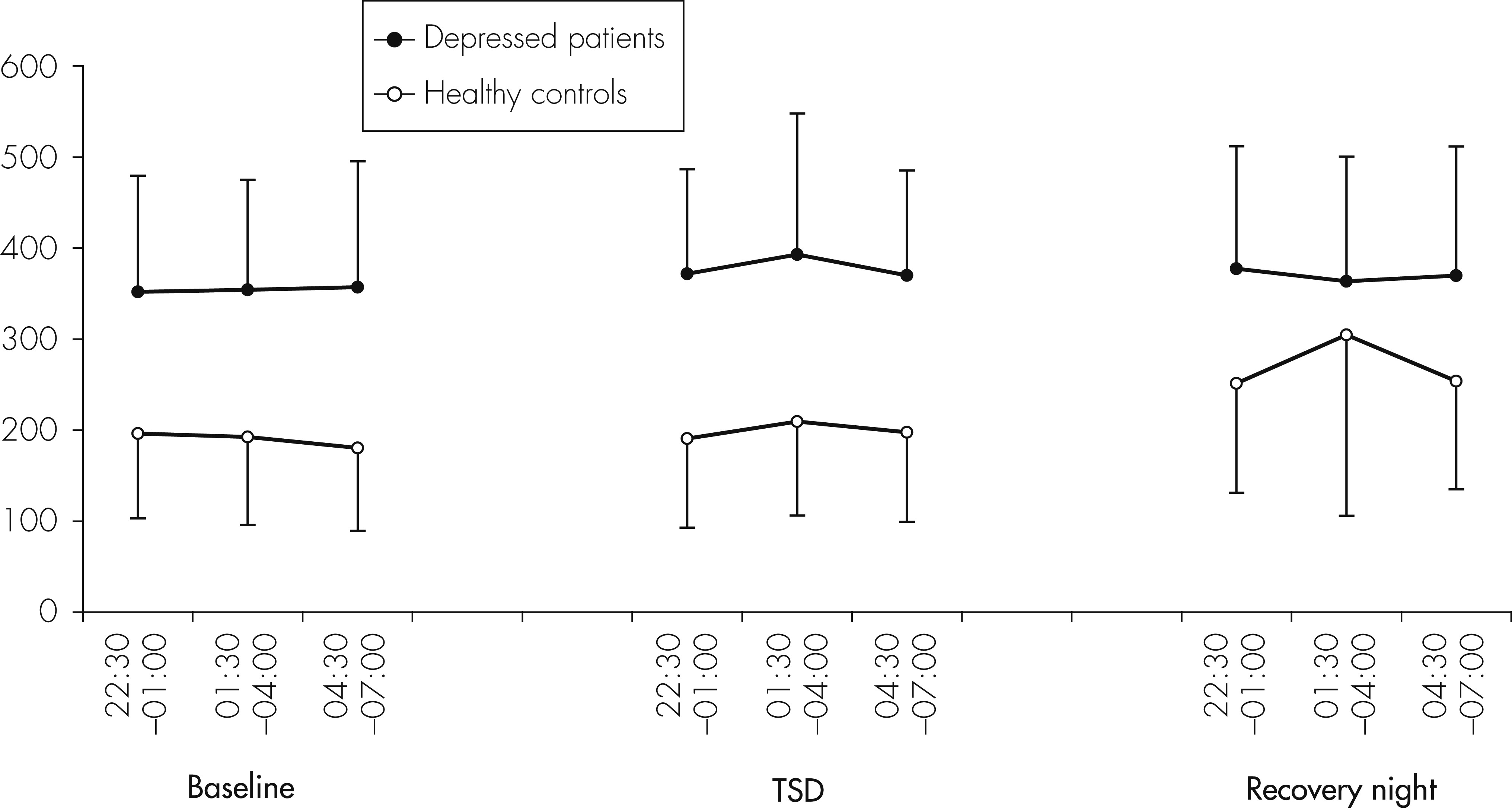
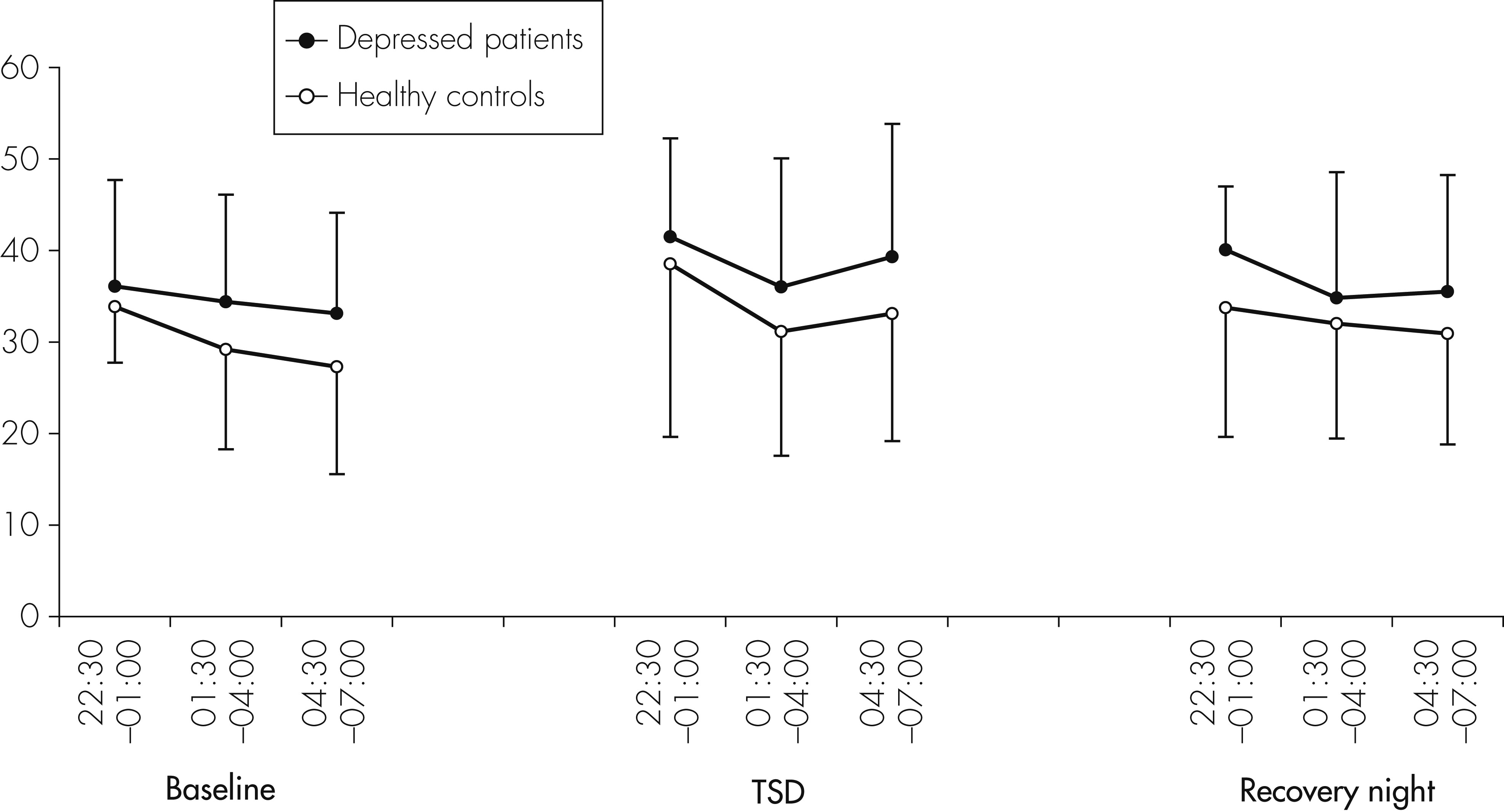
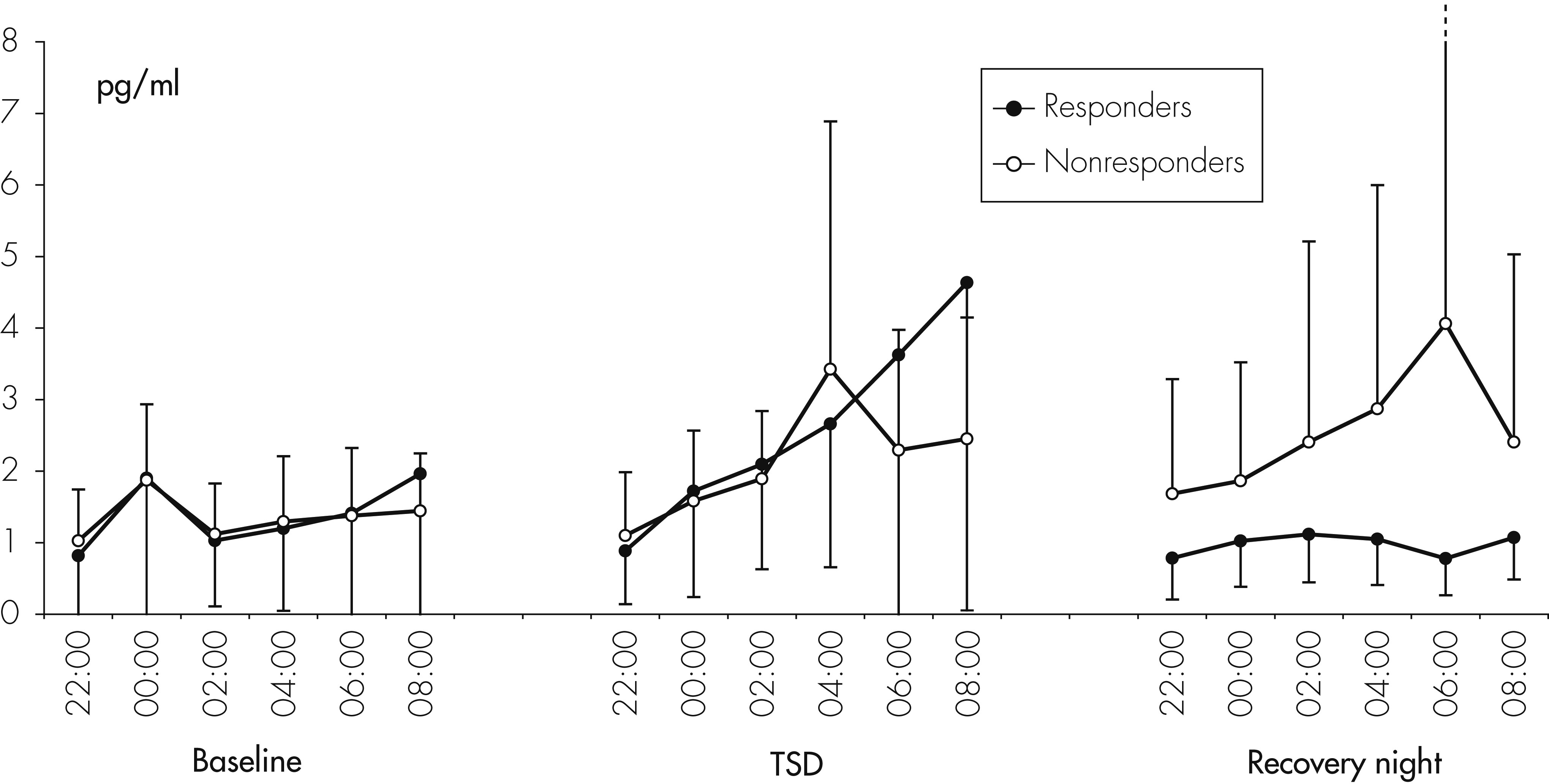
The main result of this study is a significant but complex impact of TSD on peripheral concentrations of cytokines in humans. We found a significant increase of IL-6 concentrations in both depressed patients and healthy controls during the night of TSD. With regard to IL-1RA, levels were higher in depressed patients than in controls, but did not significantly change during the three consecutive nights, whereas, in controls, IL-1RA levels increased significantly during the recovery night. This result is in line with a recent study
16 that also found elevated levels of IL-1RA in depressed patients; sIL-2R concentrations were slightly but significantly higher during TSD in both groups.
A study investigating changes in cytokine levels in sleep-deprived animals showed results comparable to our study. After 36 hours of sleep deprivation, levels of IL-6 and IL-1RA were increased in mice.
31 REM sleep deprivation over 72 hours led to elevated levels of IL-1, IL-1β, IL-6, IL-17A, and TNF-α in rats.
32 The source of elevated cytokines seem to be mononuclear phagocytes, albeit few data exists on the cellular origin.
33 Our results regarding cortisol are in line with several studies showing that sleep deprivation elevates cortisol levels.
34First of all, methodological aspects have to be discussed. Haack et al. reported that peripheral IL-6 concentrations rose within 10 hours in blood samples taken from the forearm where the catheter was placed, whereas control samples taken from the contralateral arm at identical time-points did not demonstrate such elevations.
35 Therefore, increased cytokine levels during the night of TSD might be confounded by the blood-drawing procedure itself. However, in our study, a new i.v. catheter was inserted before each of the three consecutive nights, and a strong increase of IL-6 concentrations was only observed during the night of TSD, but not during the nights when subjects slept. Therefore, it seems unlikely that increased IL-6 concentrations during TSD are due to an increased local IL-6 synthesis because of the placement of an i.v. catheter. Secondly, several previous studies only measured single serum samples from each subject. Since serum concentrations of IL-1RA, IL-2R, and IL-6 are significantly affected by age, body mass index, gender, smoking habits, ongoing infectious diseases, or previous medication,
11 and may show circadian secretion,
36 inconsistent study results may well be due to incomplete matching of patients and controls according to these possibly confounding variables. In the present study, the matching as well the assessment of cytokine levels at several measurement points make it unlikely that the results are due to findings by chance.
Several previous studies investigated changes of cytokine and cytokine-receptor levels by partial sleep deprivation (PSD) or TSD in healthy volunteers. With respect to IL-6, our results are supportive of earlier studies demonstrating increased levels of IL-6
18–21 in response to one or more nights of sleep deprivation. In contrast, our findings are not in line with studies of Dinges and coworkers, who failed to find significant changes in IL-6 levels after short-term sleep deprivation,
37 and Born et al., neither of whom found a significant change in IL-6 during TSD as compared with a control night.
38 Frey et al. and Vgontzas et al. even found decreased IL-6 levels after total sleep deprivation in healthy subjects.
25,39 The cause of the divergent results is unclear, but differences in methodology, especially the assessment of cytokine levels during day- or nighttime, may account for them. Redwine and colleagues investigated the effect of partial sleep deprivation (PSD) on IL-6 concentrations in healthy volunteers.
40 They found a nocturnal increase of IL-6 after sleep onset, which was delayed after PSD until 3 A.M. Their interpretation of this finding was that loss of sleep may serve to decrease nocturnal IL-6 levels. However, their increase of IL-6 concentrations is similar to the increase seen in our study on the night of TSD, which might indicate that the increase in IL-6 levels is not the consequence of recovery sleep after PSD, but of the sleep deprivation period before. Recently, Vgontzas et al. reported that napping during daytime after sleep deprivation
41 induced a decrease of IL-6 concentrations for several hours, which fits with our finding of an increase of IL-6 after sleep deprivation.
Contrary to IL-6, IL-1RA concentrations did not increase during one night of TSD. This is in contrast to recent studies showing increased levels of IL-1RA after 40 hours of total sleep deprivation.
22 These studies, however, did assess cytokines during daytime, which makes a direct comparison with our study difficult. In the group of healthy subjects, but not in the depressed patients, IL-1RA concentrations were significantly increased during recovery sleep. Since IL-1RA belongs to the IL-1-receptor family, and IL-1RA concentrations correlate with those of IL-1, the increase of IL-1RA after 40 hours without sleep in our healthy subjects may fit with earlier studies demonstrating an increase of IL-1 during periods of sleep deprivation.
37,42 Similarly, Born et al. found an increase of IL-1β-production during wakefulness, as compared with a control condition when subjects were allowed to sleep.
38A further finding of our study was a stimulatory influence of a night of TSD on sIL-2R concentrations. Born et al. measured the production of IL-2 during sleep and sleep deprivation and found significantly lower levels during sleep deprivation than in the sleep condition.
38 Since sIL-2R and IL-2 are inversely related, our results of a stimulatory effect of sleep deprivation on sIL-2R might be explained by a suppressive effect of sleep deprivation on IL-2.
So far, there is, at least to our knowledge, only one previous study that investigated cytokine levels in relation to TSD in patients with MDD.
23 In this study, increased IL-6 levels at the day before a night of TSD and a night of sleep-phase advance predicted a worse response to TSD in depressed patients. However, this study has many shortcomings, such as small sample size (N=10), single determinations of IL-6 levels, and unreliable ratings for treatment response. Therefore, these data can only be interpreted with caution. As far as we know, up to now, no other studies reported on the effect of sleep deprivation on cytokine levels in depressed patients. In our study, TSD led to a significant increase of IL-6 levels in unmedicated depressed patients that was reversed on the recovery night. This finding might be relevant with regard to several earlier reports on elevated IL-6 concentrations in depressed patients,
4 postulating that an activation of the immune system with a consecutive activation of the HPA system might be a relevant patho-mechanism of depression. Our results, however, might also be consistent with the idea that elevated IL-6 levels are an epiphenomenon of disturbed sleep, which is usually a part of the depressive symptomatology. This may underline the possible role of disturbed sleep as the cause of increased IL-6 levels found in several studies and may also possibly underline the role of disturbed sleep as the primary pathogenetic factor for the development of depressive disorders, a view that is supported by several epidemiological studies.
43With respect to treatment response to TSD and cytokine levels, no statistically significant differences were found between nonresponders and responders for IL-6 and sIL-2R. There was, however, a nonsignificant trend to lower IL-6 levels on the recovery night in responders compared with nonresponders. Regarding IL-1RA, a significant increase was observed in the responder group, but not in the nonresponder group. Because of the small number of nonresponders and responders, however, these small effects should not be over-interpreted, and conclusions should not be drawn from these effects until they are confirmed by a study in a larger sample of responders and nonresponders.
The underlying mechanisms of increased cytokine concentrations in response to TSD are unknown. One explanation for the elevation of IL-6 might be an increase of catecholamine levels after sleep deprivation,
38 since catecholamines have been shown to increase the production of cytokines,
44–46 and exercise-induced release of epinephrine and norepinephrine correlates with increases in IL-6.
47 Since sleep deprivation also has been shown to increase synthesis of IL-1,
21,22,48 and IL-1 is able to stimulate IL-6 in various cell types, for example, as in the findings of Lieb et al., Bauer et al., and Helle et al.,
49–51 the increased IL-6 levels during TSD might also be mediated by IL-1. However, in our studies, we were not able to detect IL-1 in our serum samples with our ELISA.
The possible functional consequences of increased cytokine levels during a night with sleep deprivation are unknown. Several issues should be discussed. It has been shown that cytokines such as interleukin-1 (IL-1) and interleukin-6 (IL-6) interact with the central nervous system by activating the hypothalamic-pituitary-adrenal axis (HPA axis).
48,52,53 Furthermore, an activation of the HPA axis can induce increased production of IL-6 by itself and can maintain cortisol production from the adrenal glands even in the absence of ACTH.
54 An activation of the HPA axis plays a key role for the response of the organism to stress, and the HPA axis is activated in acutely depressed patients. An increase of cytokines after disturbed sleep might therefore trigger an activation of the HPA axis and thus contribute to the increased risk of developing a depressive disorder after chronic insomnia.
Another aspect is the direct behavioral effects of cytokines, which induce a variety of central nervous effects, such as fever, apathy, fatigue, and sleepiness.
55,56 It has been proposed that increased cytokine concentrations after sleep disruption might partly account for the fatigue in patients suffering from insomnia.
39,57With respect to the antidepressant effects of sleep deprivation in depressed patients, it seems contradictory that a night of acute sleep deprivation elevates IL-6 and cortisol
34 but induces a transient improvement of depressive symptoms.
26 However, acute sleep deprivation in depressive patients also causes a transient but almost complete normalization of disturbed sleep on the recovery night. Vgontzas et al. reported that, in healthy subjects, acute sleep deprivation reduces cortisol secretion the next day via an increase of slow-wave sleep and hypothesized that this might also be the mechanism through which sleep deprivation relieves depression temporarily.
41 This indicates that the neurobiological effects of acute sleep loss and chronically disturbed sleep may not be the same. In a study of modest sleep restriction from 8 to 6 hours/day for 1 week, IL-6-secretion was increased, whereas peak cortisol secretion was decreased in 25 healthy volunteers.
58 Accordingly, a study investigating a more severe sleep restriction to 1 hour/day for 1 week showed similar results, with increased IL-6 levels as a result of an increasing level of activity of leukocytes over the study period, but normal cortisol levels.
59 These results show that chronic sleep deprivation leads to increased IL-6 levels, which are also seen in depressed patients.
4–8 Inflammatory cytokines interfere with many pathological findings in depression, for example, glial degeneration, altered neurotransmitter release and uptake, neuroendocrine functioning, and neural plasticity.
60–62 In summary, our study demonstrates that acute sleep loss may significantly influence peripheral concentrations of cytokines differently in healthy and depressed subjects. With regard to earlier findings in terms of elevated cytokine levels in unmedicated depressive patients, we assume that changes in cytokine levels accompanying psychiatric illness could well be secondary to a severe sleep disorder, rather than reflecting a primary pathogenic mechanism. Our results further indicate that elevated levels of cytokines such as IL-6 may partly explain fatigue and sleepiness occurring after sleep deprivation. The interaction between antidepressant effects of TSD and alterations of cytokines warrants further investigation.