Acetaldehyde, the main metabolite of ethanol, has been shown to be able to bind covalently to liver proteins and may be responsible for the diverse toxic effects of ethanol. Acetaldehyde reacts with proteins via the ε-amino group of a lysine residue to yield hybrid malondialdehyde−acetaldehyde−protein malondialdehyde and 4-hydroxy-2-nonenal alcohol metabolites. These substances induce severe metabolic alterations and cause oxidative damage, including mitochondrial DNA damage, through the production of reactive oxygen species, in almost all mammalian tissue.
In their evolution, both etiologies (i.e., the alcoholic one and the viral one) often cause severe complications, such as the development of portal vein hypertension in the splanchnic area, and consequently, the presence of a hyperdynamic systemic circulation, as well as neuropsychiatric dysfunction.
Glucose is fundamental to develop a normal function of the brain. Basically, glucose comes in adequate amounts from liver glycogen hydrolysis. The cirrhotic liver is characterized by the destruction of an important quantity of hepatocytes, where glycogen is synthesized and stored, causing a deficient nutritional condition. It is probable that, in this deficient nutritional condition, other sources of glucose (e.g., muscles) are not satisfactory enough to cover glucose needs.
Numerous authors have searched for an efficient battery of psychological tests to distinguish mild cognitive impairment. A desired battery of psychological tests should combine convenience, practicability, sensitivity, specificity, low cost, and a high probability of standardization.
In this study, the neuropsychological profile of cognitive impairment associated with chronic liver disease was characterized by applying suitable psychological tests with the purpose to evaluate the intellectual function in this type of patients.
The aim of this experiment was to recognize cognitive dysfunction present in chronic liver disease, produced by prolonged alcohol ingestion or by hepatitis B and C virus infections, from the results obtained in the tests applied to patients with either etiology.
Discussion
The clinical presentation of hepatic encephalopathy in patients with chronic liver disease, especially in decompensated cirrhosis, includes alteration of consciousness, accompanied with loss of facial expression and speech disturbances together with a compromised motor system, represented by asterixis, tremor, increased tendon reflexes, increased muscle tone, and ataxic gait. In contrast, patients with minimal hepatic encephalopathy appear to have a normal mental state but subclinical cognitive alterations that could complicate their normal day-to-day tasks such as driving, operating equipment, and work-related activities.
Cognitive dysfunction is considered the failure of certain mechanisms such as understanding, coordination, spatial perception and reality, and problem solving. These limitations occur in the patient’s personal, social, and work life to a greater or lesser degree. In turn, it is possible that the progression of cognitive impairment in life has emotional consequences for the patient, who, because of his/her limitations, may develop depressive symptoms, inhibitions, and/or impaired volition. All nerve cells are targets of alcohol and its metabolite agents and viral toxins, producing functional alterations. However, patients with a history of prolonged alcoholic intake showed more severe intellectual derangements than patients with chronic viral liver disease, except for the Reitan’s test. It can be considered that brain function impairment is caused by prolonged alcohol ingestion or long-term viral damage, derived into cirrhosis and portal hypertension.
20 These agents induce alterations in several brain structures and cellular pathways, causing mild or important cognitive defects.
The detailed psychometric or neuropsychological examination carried out in this study revealed important cognitive impairment in most of the 43 patients with liver disease.
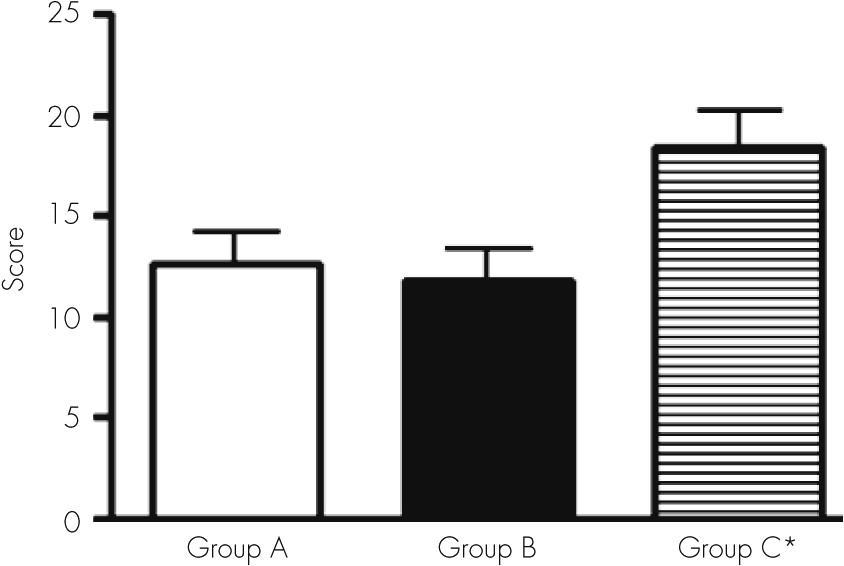
The most outstanding findings were failures related to the constructional ability and copy capacity (recognition as identical or nonidentical designs). We found an important number of mistakes in recognition in the cube and scanning tests.
The use of this group of tests allowed documenting the existence of very poor performance in the visual motor area, corresponding to coordinated functions between visual motor and spatial areas. These findings are related to a serious deterioration in the velocity (speed) of mental processes during perception and discrimination of objects.
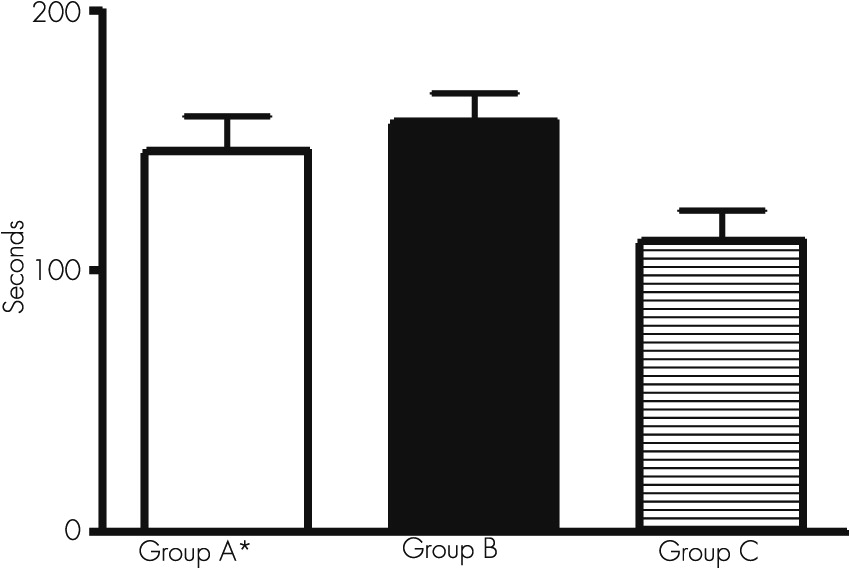
An alteration in the conjugated ocular movements during the act to follow an object can be suggested to have a diagnostic value during the presence of a subclinical hepatic encephalopathy.
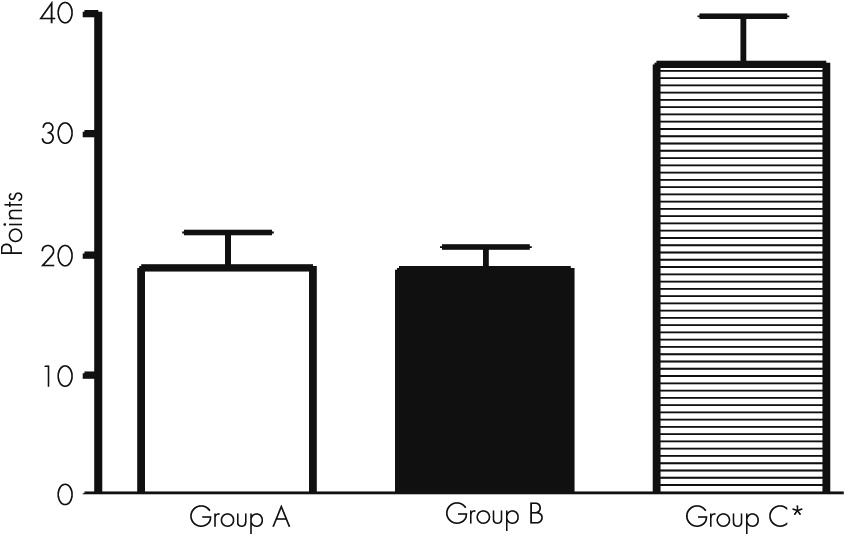
The alteration in the similarity test indicates the presence of a marked defect to conform categories, suggesting an altered capacity of comprehension and a defect in the associative capacity in verbal concept that implies a deficiency in conceptual judgment.
Chronic alcohol ingestion acts on numerous pathways that affect the function of the liver and other organs, including the brain.
10 The main metabolite of alcohol, acetaldehyde, and several adducts, malonaldehyde and 4-hydroxy-2-nonenal, damage cell structure and organelles in the liver and brain, affecting microtubular proteins and inhibiting microtubule polymerization. It has been suggested that during this damage, excessive generation of reactive oxygen species overpasses the antioxidant system, forming the so-called oxidative stress. Reactive oxygen species alter membrane structure, lipid molecules, and DNA, producing membrane lipo-oxidation and affecting lipid metabolism.
Taken together, these findings suggest that alcohol intake induces oxidative stress and constitutes a pivotal factor in the development of liver and brain injury.
Viral damage, like hepatitis B and C viruses, probably uses a similar mechanism to produce intellectual deficits through its own toxins. The presence of hepatitis B or C virus in the patient may act on brain tissue by either the action of viral toxic substances or the presence of cytokines or chemokines released by macrophages or other cells, which are mainly the liver Kupffer cells. These two factors may act on brain nuclei, causing functional impairment.
21 This speculation is based on the fact that infection induces neuroinflammation and brain dysfunction. Fatigue, mood disturbances, and cognitive dysfunction are frequent in patients infected with hepatitis C virus who have mild liver disease. The pathological mechanism is still unclear. Viral sequences and proteins have been previously found in brain macrophage/microglia cells. Bokemeyer et al.
21 found glial activation and macrophage infiltration in chronic inflammation and virus infections of the brain, endorsing the hypothesis that hepatitis C virus infection may induce neuroinflammation. Wilkinson et al.
22 also found evidence for activation of brain macrophages/microglia cells in autopsy brain tissue from hepatitis C virus-positive patients.
Some of these mechanisms can be studied using animal models. However, the extrapolation of the findings, from animals to humans, remains a difficult task, because reactive oxygen species production and antioxidant status in humans is affected by severe nutritional environmental defects and drug influences, which are difficult to reproduce in animals.
Nevertheless, several investigations have found that the administration of antioxidant agents that reduce the levels of free iron or of agents that replenish glutathione levels can prevent or ameliorate the toxic effect of alcohol. The most convincing data, which indicate that oxidative stress contributes to alcohol liver disease, have come from studies using the intragastric alcohol infusion model. In these studies, alcohol liver disease was associated with enhanced lipid peroxidation, protein modification, formation of the 1-hydroxy-ethyl radical and lipid radicals, and a decrease in the hepatic antioxidant defense, particularly glutathione levels.
23–25 Moreover, changes in the animal diet promote or reduce oxidative stress, leading to the corresponding changes in the extent of liver injury, as occurs when polyunsaturated fats, which are required for lipid peroxidation production, are replaced with saturated fatty acids
26 and perhaps on brain damage also.
According to previous experiments from our laboratory, brain regions, specifically the hippocampus and prefrontal region, are involved in metabolic, functional, and morphological derangements in a portal hypertensive rat model, which reproduces this major cirrhotic complication using a simple stricture of the portal vein.
27The hippocampal region is a brain median temporal lobe formation that takes part in the processing of memory, behavior, and learning. It is also related to other parts of the brain, such as the brain cortex and amygdala. Thus, this brain region is probably involved in brain damage by different toxins and possibly participates in intellectual derangements.
Rusakov et al.
28 studied the correlation of spatial learning in the rat hippocampus with synaptic ultrastructural appearance and suggested that it is possible to recognize brain areas and even cell populations in particular learning paradigms.
Stubley-Weatherly et al.
29 examined the effects of hippocampal lesions using water maze performance in rats and found that both the dorsal CA1 and ventral CA3 subregions play important roles in learning.
Rats maintained in a spatially enriched environment show improved performance in a water maze task, which is associated with an increased density of dendritic spines in CA1 pyramidal cells, indicating an increased number of newly formed synapses associated with spatial learning. These results provide evidence that memory formation, at least in rats, may involve morphological and topographic changes in some neural circuits.
Unexpectedly, the memory function, analyzed for a short time or long time, shows minor alterations when comparing cirrhotic patients with control subjects, but visual memory was affected.
4,5,30 Perhaps the lack of a significant failure in memory can be due to lesser damage to the hippocampus region. It would be interesting to study hippocampus behavior and its basic influence on memory in alcoholic and nonalcoholic chronic liver disease.
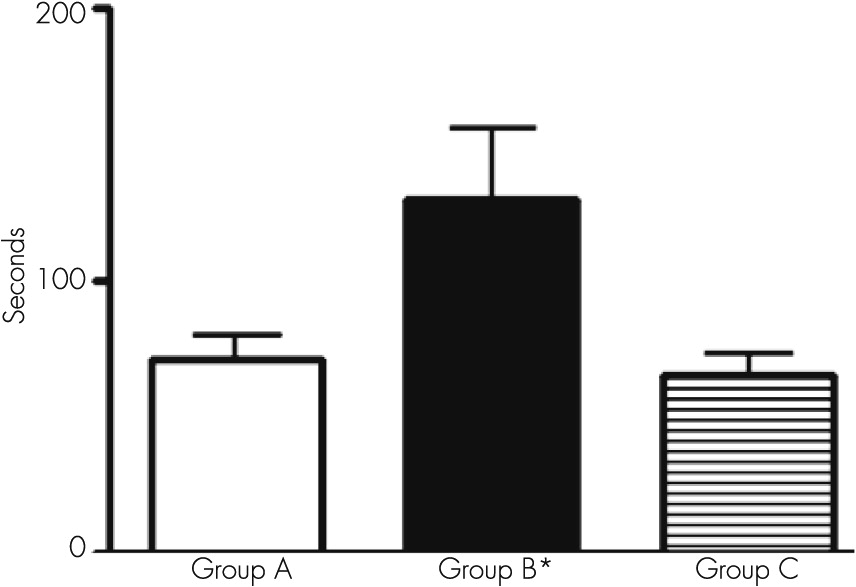
When we used the Boston Naming Test with our patients, we observed that it took a significantly long period of time for the patients to complete the answer, indicating the presence of a slower mechanism of thinking (patients must observe the object to remember its name). This kind of behavior is called paraphrasis and demonstrates the capacity of the patient to scan the information. Retrograde semantic memory is involved in this procedure. During depression, this test can show a normal result, but in dementia, scarce verbal fluency can be present.
The analysis of our results, after the application of this battery of tests, demonstrated that patients with chronic liver disease, from either alcoholic intake or viral damage, had an important impairment in their attention and mental concentration. Interestingly, mental alterations were documented when tests were applied to involve the praxis aspects (motor activity) and those that evaluate the velocity of the mental processes.
The psychoneurological symptoms that appear during chronic liver disease may correspond to the presence of several and complex mechanisms. Peterson et al.
14 and Lozeva et al.
31,32 reported that neurotransmitter systems, particularly alterations in the glutaminergic and monoaminergic receptors, are implicated, as shown in brains of cirrhotic patients and experimental models.
33The role of these pathways is to mediate important brain functions, such as the regulation of the sleep-wake cycle and the act of learning and memory.
Weissenborn et al.
4 showed that, as the degree of cognitive impairment in cirrhotic patients without clinical signs of hepatic encephalopathy increases, the glucose use of the frontal and parieto-occipital cortex decreases. Because the brain metabolism of glucose is closely related to liver glycogen, the results of Weissenborn et al. are very interesting.
Cirrhotic liver and its scar and nodular formation decrease the number of normal hepatocytes and their function, independently of the etiology: alcohol or toxins from virus. On one hand, less glycogen is stored, with the possibility of sending less glucose to the brain; on the other hand, as occurs in long-term alcoholic consumption, there is a decrease in glutathione levels in the mitochondria.
The neuropsychological tests that are used in this research confirmed the presence of cognitive impairment in patients with liver cirrhosis due to alcoholic prolonged intake or hepatitis B or C virus damage. The presence of oxidative stress must be assumed in these patients as part of the mechanism of cognitive alterations. According to the literature, 51%−70% of cirrhotic patients present cognitive alterations. Animal models exposed to ammonium acetate show an important decrease in the activity of glutathione peroxidase in the liver and brain, which is the same as liver peroxidation. Ammonia is toxic to brain function and is the result of urea metabolism in the damaged liver that is caused by cirrhosis.
Corbalán et al.
34 found that the nitric oxide-guanylate cyclase signal transduction pathway is altered in brains of patients with chronic liver damage and that these alterations are different depending on the brain area involved.
An increase in Ca2+ intracellular concentration, which stimulates glutamate receptors (N-methyl-d-aspartate), can also modify the signal transduction pathway in the brain. The entry of Ca2+ into CNS cells triggers the production of nitric oxide by neuronal nitric oxide synthase activation. This molecular alteration can produce different levels of intellectual changes, with coma being the most serious event in cirrhotic patients.
Injection of cyclic GMP, a hyperglycemic agent, causes an increase in brain glucose, ameliorating brain function.
According to the results by Erceg et al.,
35 the intracerebral injection of cyclic GMP to chronic hyperammonemic rats restores the ability to learn and memorize, using a Y-maze conditional discrimination task. The administration of an anti-inflammatory drug, such as ibuprofen, also contributes to the amelioration of learning, using the same Y-maze.
36 We then suggest that both cyclic GMP and ibuprofen act on the impairment of the glutamate-nitric oxide-cyclic GMP pathway in the brain and, by different mechanisms, restore learning capacity.