Impulsiveness is a common component of psychiatric and neurological illness,
1 present to a pathological degree in schizophrenia, bipolar disorder, and certain neurological disorders, including the progressive neurological disorder sometimes seen in boxers, known as “punch-drunk syndrome,” or dementia pugilistica.
2 At autopsy, these disorders related to boxing will often show signs of chronic traumatic encephalopathy (CTE
3), a disorder that also occurs as a result of repetitive head injuries in contact sports. Although elevated impulsiveness is described in dementia pugilistica,
2 there have not been any studies assessing impulsiveness in active fighters. Given the scheduled and, in many ways measurable, repeated head injuries seen in these athletes, they provide an obvious model for assessing behavioral manifestations of this kind of trauma.
Boxers and mixed martial arts (MMA) fighters with high levels of exposure to their sports (i.e., more fights, or more years of fighting) are found to have reductions in the volume of various brain structures, notably the thalamus, hippocampus, and caudate (Bernick C, et al., The threshold effect of repeated head trauma on the brain; unpublished; and Bernick C, et al., 2012
4), consistent with gross atrophy found at pathology in confirmed cases of CTE.
5 Given the involvement of these structures in important frontal–subcortical and limbic circuitry, it is plausible that their damage might promote impairment in emotion-regulation and, hence, higher levels of impulsiveness. Indeed, even small neurosurgical lesions to the subthalamic nucleus have been associated with increased impulsiveness,
6 as have small lesions to the amygdala.
7 Although any damage sustained to these structures during professional fighting would be more diffuse, it is reasonable to foresee a relationship between damage to the basal ganglia structures or the amygdala and impulsiveness.
The hypothesis of interest driving this study was that there would be a relationship between higher levels of exposure to boxing and MMA and elevated levels of impulsiveness. Furthermore, this impulsiveness was expected to be related to reduced volume of various structures, most likely the thalamus, amygdala, and hippocampus.
Methods
The overall methods for the PFBHS are detailed elsewhere,
8 and therefore are only briefly outlined here.
Data Collection: Barrett Impulsiveness Scale, Demographics, and Fight Exposure
Participants completed a structured interview to provide demographic data and fight-exposure data. Variables of interest included participant’s age, total number of years of education (YOE), total number of fights (NOF), total number of years fighting (YOF), and the average number of fights per year (FPY; i.e., NOF/YOF). Number of fights was self-reported, but were then checked on
Boxrec.com for professional boxers and the MMA websites
mixedmartialarts.com and
Sherdog.com.
The BIS II comprises 30 questions, which have been shown to load on six factors; the items loading on these factors provide the subscales for the instrument. Individual questions can be scored either forward or in reverse. For both total and subscale scores, higher scores refer to higher levels of impulsiveness. The Attention subscale asks questions about how easily the person concentrates versus how restless and fidgety he is. The Cognitive Instability subscale refers to racing thoughts, changing of hobbies, and extraneous thoughts while thinking. The Motor subscale refers to actions—doing things quickly and without thinking, including acts in general and purchasing behavior. The Perseverance subscale refers to changing jobs and residences frequently, thinking about more than one thing at a time, and being future-oriented. Questions on the Self-Control subscale refer to planning and being careful, and the Cognitive Complexity subscale refers to working through problems, saving for the future, and interest in the present versus the future.
Data Collection: Neuroimaging
MRI scans were acquired on a 3T Verio scanner, with a 32-channel head coil. Volume of the subcortical gray matter, including hippocampus, amygdala, thalamus, caudate, putamen, and pallidum were calculated on anatomical T
1-weighted image, using the automated full-brain segmentation process in Freesurfer software.
9 The subcortical volume in the regions of interest (ROIs) were measured in each hemisphere separately.
Data Analysis
We assessed the distribution of the impulsiveness scores, using Q–Q plots and assessments of kurtosis and skewness. The distributions of the outcome variables were near normal; thus, no transformations to the data were considered.
We first compared the mean impulsiveness total and subscale scores of the 131 study subjects with the published mean scores of 393 male controls
10 by use of a Wald test.
Generalized linear models were constructed to assess whether fight exposure was predictive of impulsiveness scores, after adjusting for age, race, and education. Separate models were built for the total score and each subscale. The dependent variable in each model was the Impulsiveness score; fight exposure was the independent variable of interest. Type of fighter (boxer or MMA fighter) was included in each model. The interaction between fight exposure (total number of fights, total number of years of fighting, and number of fights per year) and type of fighter was first assessed; if the interaction term was not significant, then it was removed from the model.
Brain volume was also assessed as a predictor of impulsiveness scores. Generalized linear models were constructed, where the dependent variable was the Impulsiveness score, and brain volume was the independent variable of interest (separate models for the thalamus, hippocampus, caudate, putamen, and amygdala, and the volume of the structures on the left and right were averaged for analysis). The interaction between brain structure volume and the type of fighter was also assessed.
All analyses were adjusted for age (treated as a continuous variable), education (defined as no college-level versus some college-level), and race (defined as: 1) Caucasian; 2) African American; and 3) other [Asian, Pacific Islander, American Indian, and Alaskan Native]). Least-squares means of impulsiveness scores, adjusted for age, race, and education, were reported.
Impulsiveness scores were collected on 6 female fighters. We felt that the associations between impulsiveness and fight exposure could differ by sex, but with only 6 female fighters, we do not have sufficient power to model sex-specific effects. Thus, we decided to omit the female fighters from this analysis.
A significance level of 0.05 was used to test the significance of the regression coefficients of the exposure variables. All analyses were performed with SAS 9.2.
Results
Participants
The 131 study subjects ranged in age from 19 to 71, with a mean age of 28.5 years; 35.9% of study subjects were Caucasian; 32.1%, African American; and 32.1%, Other; 49.6% of subjects had more than a high school education (mean years of education: 13.5 years). There were 60 boxers and 71 MMA fighters. The fight exposure of the study participants, by type of fighting, is summarized in
Table 1.
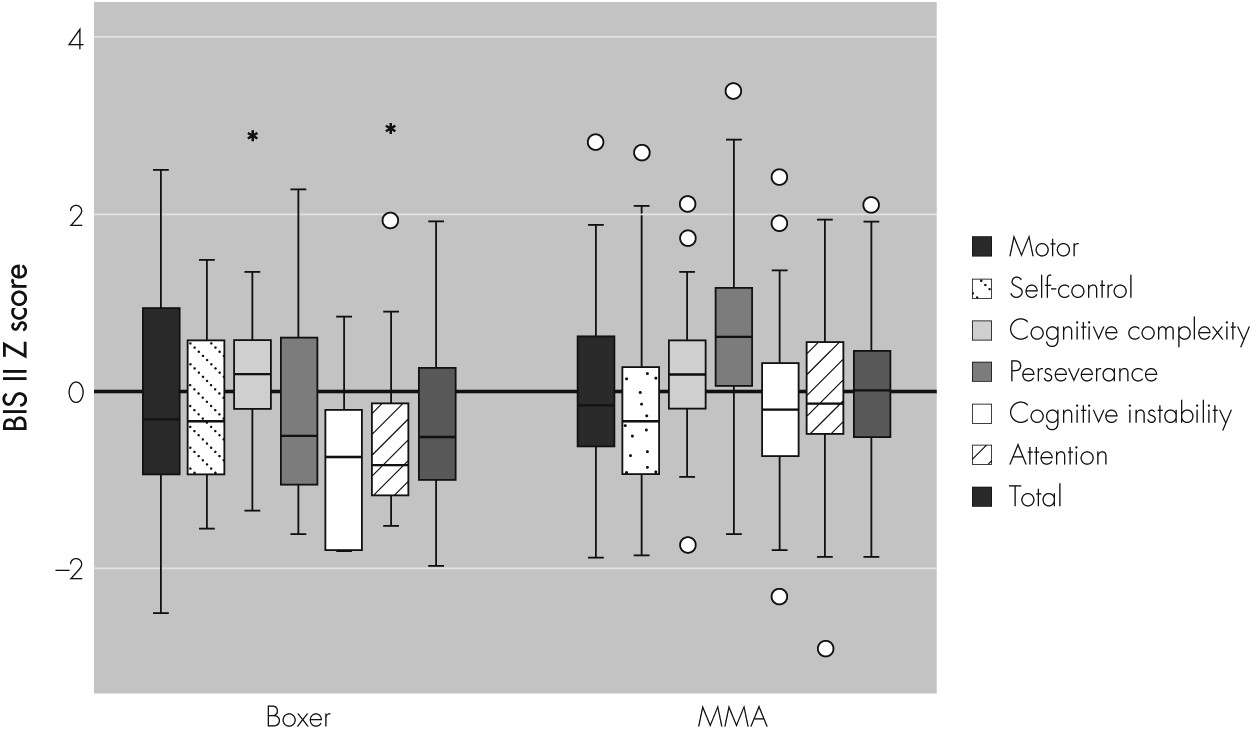
A control group of healthy male adults and college students, with an age range of 17–45 and an average of 13.4 years of education, was identified. Compared with this control group, the study sample had significantly lower Total scores (60.5 versus 62.8; p=0.012), Self-Control scores (11.5 versus 12.4; p=0.032), and Cognitive Instability scores (5.3 versus 6.4; p <0.001), and significantly higher Cognitive Complexity scores (12.3 versus 11.3; p <0.001). The spread of scores depicted as Z scores (i.e., compared with a normal sample [Stanford et al., 2009
10] and standardized to a mean of 0 and standard deviation of 1) is illustrated in
Figure 1.
The BIS II scores tended to be higher for MMA fighters than for boxers. MMA fighters had significantly higher scores than boxers for the Total score (p=0.004), the Attention subscale (p=0.002), and the Cognitive Instability (p=0.003) subscale. The mean BIS II scores, adjusted for age, race, and education, are summarized in
Table 2 by type of fighting activity.
Relationship of BIS II and Subscores With Brain-Structure Volumes
The caudate volume was significantly associated with the total BIS II score, the Attention subscale, the Cognitive Complexity subscale, and the Self-Control subscale. As the caudate volume decreased, the total BIS II score (p=0.025), the Attention score (p=0.042), and the Self-Control score (p=0.008) increased; this effect was similar for boxers and MMAs. In contrast, as caudate volume decreased, the effect on Cognitive Complexity scores depended on the type of fighter (p=0.001), decreasing for boxers (p=0.005) and increasing for MMAs (p=0.010).
Thalamus volume was significantly associated with the Attention, Cognitive Complexity, Motor, and Self-Control subscales. As thalamus volume decreased, Attention scores increased (p=0.039), as did Self-Control scores (p=0.027), but Motor scores decreased (p=0.036); the effect was similar for boxers and MMAs. In contrast, as thalamus volume decreased, the effect on Cognitive Complexity depended on the type of fighter (p <0.001), decreasing for boxers (p=0.002) and increasing for MMA fighters (p=0.027).
Hippocampal volume was significantly associated with Cognitive Complexity and Self-Control subscale scores. The effect of hippocampal volume on Cognitive Complexity differed depending on the type of fighter (p=0.025); as hippocampal volume decreased, the Cognitive Complexity subscale scores were not affected for boxers (p: NS), but increased for MMA fighters (p=0.006). The Self-Control subscale scores increased with decreasing hippocampal volume similarly for boxers and MMA fighters (p=0.029).
Putamen volume was significantly associated with Cognitive Complexity subscale scores, but the effect differed for boxers and MMA fighters (p=0.009). As putamen volume decreased, the Cognitive Complexity subscale scores decreased for boxers (p=0.032) and tended to increase for MMA fighters (p=0.057).
Exposure Measures and Brain-Structure Volumes
These data are published elsewhere.
4,5 To summarize: the relationship of exposure on the left and right hemispheres differed very little; there were few relationships between brain-structure volumes and exposure metrics in fighters with less than 5 years of experience, but fighters with 5-or-more years’ experience showed significant reductions in caudate and amygdala volume and a trend toward reductions in thalamus and putamen volume. Of note, boxers’ thalami and hippocampi were significantly smaller than those of MMA fighters.
Effect of Exposure Metrics on BIS II
The total number of fights and the total number of years of fighting were significant (although not independent) predictors of the Attention subscale score; the effect of exposure differed for boxers and MMA fighters (p=0.012 and 0.002, respectively, for the interaction of type of fighter and exposure). For boxers, for each increase of 10 fights, the Attention score tended to increase by 0.13, on average, (p=0.066) and, for each increase of 10 years of fighting, the score tended to increase by 1.1 (p=0.067). In contrast, for MMA fighters, for each increase of 10 fights, the Attention score tended to decrease by 0.10, on average, (p=0.081) and, for each increase of 10 years of fighting, the score decreased by 1.5 (p=0.020).
Similarly, the total number of years of fighting was a significant predictor of the Cognitive Complexity subscale scores, but the effect differed by the type of fighter (p=0.015). Boxers demonstrated a decline in Cognitive Complexity scores as the total number of years of fighting increased (0.9 decrease, on average, for each 10 years’ increase in fighting; p=0.029), whereas MMA fighters showed a trend toward increased score (1.1 increase for each 10 years’ increase in fighting time; p=0.059).
The total number of fights was a significant predictor of the Motor subscale scores. As the total number of fights increased, the Motor subscales increased (0.1 increase, on average, for each increase of 10 fights; p=0.026); the effect was similar for boxers and MMA fighters.
Discussion
This study of active professional fighters explores the relationship between exposure to cumulative head trauma and brain-structure volumes and impulsiveness. We anticipated that increasing fight exposure would be associated with accumulating brain damage as measured by MRI volumetric loss and expressed as increasing impulsive behavior.
Participants in the PFBHS reported less impulsiveness, as compared with a group of non-fighting healthy controls, as measured by the Total score and subscales assessing Self-Control and Cognitive Instability, whereas they reported higher levels of impulsiveness in Cognitive Complexity. There was an interesting difference between MMA fighters and boxers, with the MMA fighters reporting more impulsiveness than boxers on several subscales.
We have shown that increasing exposure to professional boxing and MMA fighting is related to reduced volume of the caudate and amygdala, and a trend toward reduced volume in the thalamus and hippocampus. In the current study, we demonstrate relationships between self-reported impulsiveness and exposure. More fighting was consistently associated with higher levels of motor impulsiveness (doing things quickly and without thinking; acting on impulse). In boxers, more exposure was also related to higher scores on the Attention scale (ability to concentrate).
The volume of several brain structures correlated with impulsiveness scores for both groups of fighters: Reductions in caudate and thalamic volume were associated with higher scores on the Attention scale, as well as the Self-Control scale. Smaller caudate volumes were also related to higher levels of impulsiveness overall. Smaller hippocampal volumes were associated with reduced self-control.
There were differences between boxers and mixed martial arts (MMA) fighters in the pattern of relationships between impulsiveness, brain-structure volume, and exposure: whereas boxers with more fight exposure reported more impulsiveness on the Attention subscale, MMA fighters reported less. These patterns were not reflected in the brain-structure volume data. Boxers with more fight exposure reported less impulsiveness on the Cognitive Complexity subscale. Boxers also differed from MMA fighters in the relationship between caudate, thalamic, and hippocampal volumes and Cognitive Complexity (e.g., working through problems; saving for the future), with boxers showing less impulsiveness on this score to be associated with smaller structures, and MMA fighters showing the opposite relationship.
Fighters in the current study tended to report less impulsiveness than non-fighting controls. A possible explanation is that fighters have reduced insight into impulsiveness. Indeed, patients with moderate-to-severe brain injuries have been shown to rate themselves as less impulsive, as compared with ratings made by their family members.
11 Future research, with a control group well matched on demographic factors to our sample, and longitudinal research with our sample, may help clarify whether the fighters in this study truly differ from their peers in self-reported impulsiveness, and whether this changes with more fights.
If the patterns seen in this cross-sectional study are realistic, we would expect to see increases in Motor impulsiveness (e.g., acting on impulse or on the spur of the moment) over time in the longitudinal study, and if the relationship between fight exposure and brain-structure volume is causal, we may see increases in overall impulsiveness, ability to concentrate, and ability for self-control over time, with a relationship with reduced caudate volume.
Although Motor impulsiveness increased with more fights, smaller thalamic volumes were associated with lower levels of Motor impulsiveness. This finding is consistent with that of Antonucci and colleagues,
12 who found larger orbitofrontal cortex volume to be associated with more motor impulsiveness in psychiatric patients. The meaning in the context of the current study is unclear.
When the cohort was divided into types of fighting, differences in impulsiveness between the MMA fighters and boxers were apparent. MMA fighters had higher levels of impulsiveness overall, and higher scores on the Attention and Perseverance subscales. The reason for the differences seen in impulsiveness between MMA fighters and boxers could be a question of more open responding, or simply a different personality type being attracted to the two sports, but certainly this finding warrants further research.
There was a surprising difference between boxers and MMA fighters in the relationship between their caudate, thalamus, and hippocampal volumes and scores on the Cognitive Complexity scale: this relationship went in opposite directions for the two groups, with smaller volumes related to higher levels of impulsiveness for the MMA fighters, but reduced impulsiveness in the boxers. This scale relates to analytical thinking—a higher cognitive skill. The pattern might be explained by overall differences in volume of these brain structures seen between the two groups, with MMA fighters having larger structures and perhaps more to lose in the early stage of their careers. Longitudinal data will determine whether reductions in fighters’ brain-structure volume has an impact on this scale in within-group analyses.
We expected to find an association between amygdala volume and impulsiveness in this study. The absence of such a relationship is particularly surprising, given the robust finding of exposure related to reductions in amygdala volume and the known role of the amygdala in emotional control.
Several limitations of our study need to be recognized. One is a lack of a non-fighting control group who went through the same tests as the fighters in this study. We do not know the relationship between the volumes of the brain structures discussed here and impulsiveness in the general population. Although we were able to compare the fighters’ BIS II data to published control data, we are currently recruiting a healthy-control group, but, at present, no data are available for comparison. With regard to measuring exposure, although the professional records of each fighter are verified by industry organizations, amateur records are self-reported. The relationships seen here between BIS II subscales and exposure and neuroimaging data were inconsistent, suggesting that using the overall score may not be appropriate in this population, and further research is necessary to understand the relationship between fight exposure and the subscales.
Further analysis of the neural underpinnings of impulsiveness in this population is warranted, and we will conduct further research, using diffusion tensor imaging to assess the integrity of white matter, as well as resting-state functional MRI to assess for functional connectivity changes that might be related to changes in impulsiveness.
The current results suggest several questions about the relationship between professional fighting and impulsiveness. Further study of this under-researched topic may have important implications both in understanding brain–behavior relationships and in making recommendations for the long-term psychological health of these athletes.