Single-photon emission computed tomography (SPECT) is a well-established nuclear medical imaging technology thought to show functional activity indirectly via measurement of relative perfusion. Visualizing the different levels of activity in the brain may allow more accurate evaluation of psychiatric disorders by revealing abnormally functioning neural regions and circuits, hence allowing more targeted, effective treatment.
1–5 For example, the finding of increased regional cerebral blood flow (rCBF) in the subgenual cingulate and prefrontal cortex demonstrated by SPECT predicted which depressed patients would more readily respond to citalopram.
4In recent years, the analysis and visual representation of SPECT, which we term “3D SPECT,” has been shown to facilitate the management of psychiatric patients.
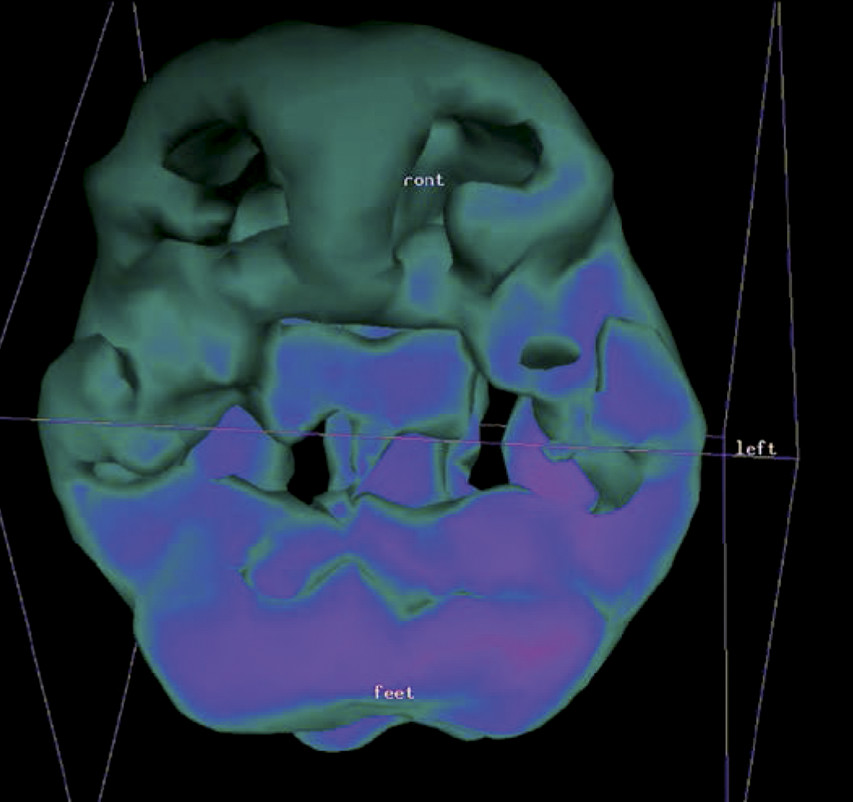
1,3,6 3D SPECT processes the scanning data via thresholding functions to synthesize a 3D model of the brain. (See
Figure 1 and
Figure 2.) Pixels representing the top 45% of activity are used in creating the outside cortical 3D surface view of the brain, and the top 8%, 15%, and 45% pixels create the three-dimensional view of the interior of the patient’s brain. These thresholds reflect the early work of I. Mena and colleagues
7–9 and have been used by Amen
6 in tens of thousands of SPECT scans. The term “3D SPECT” herein refers to the production of such SPECT images.
The literature shows that for particular disorders, the defects on SPECT scans correlate with the clinical deficits. For example, Ichise and colleagues
10 have shown, in traumatic brain injury, a correlation between brain SPECT defects and neuropsychological performance. However, in a full-spectrum, community-based psychiatric practice, does an objective scoring of before-treatment and after-treatment SPECT scans correlate with an objective measure of patient functioning before and after treatment? Another question to be answered is a clinically important one: Is 3D brain SPECT scanning a useful procedure for the community clinician? Do patients who receive SPECT scans and SPECT-guided treatment improve more in final functioning than matched-control patients who receive treatment-as-usual that does not involve SPECT scans?
Methods
Brain SPECT scans were offered to patients at a community-based psychiatric clinic. Patients at this clinic had problems with anxiety and depression, as well as frequently subtle cognitive dysfunction as a consequence of injury or chronic pain, which had not responded successfully to primary-care intervention. No attempt to select patients was made. As scanning slots were made available at the local hospital, they were offered to patients in the clinic. Only a few patients each month could be scanned. Thirty patients, without regard to diagnosis, age, severity, or other factors, were offered SPECT scans in the context of routine clinical care. As part of the clinical evaluation, Global Assessment of Functioning (GAF) scores were obtained before and after treatment, as per the guidelines of the American Psychiatric Association DSM-IV-TR.
11The SPECT scanning was performed according to the guidelines established by the Society of Nuclear Medicine.
12 Patients received an intravenous injection of the 99m-technetium-labeled brain perfusion agent ethyl cysteinate dimer (ECD; 15–20 mCi) in a sitting position in a quiet, dimly-lit room. The uptake of ECD in the brain is complete in less than 90 seconds. Technetium-99m is a metastable nuclear isomer of technetium-99 with a half-life of 6.01 hours. The use of 99mTc-labeled brain perfusion agents has been well established over the last two decades.
13,14 Approximately ½ to 1 hour after injection (so as to allow clearance of 99m-Tc-ECD from non-neural tissues during this time) the patient’s head was scanned with a Picker Prism 3000 three-headed camera; collimation: fan beams; matrices: 128 × 128; acquisition: 120 frames over 360 degrees; attenuation correction: calculated Chang first-order attenuation correction; processing: iterative, ordered subsets expectation maximization (OSEM) reconstruction; masking: image voxels outside the head/brain region were set to 0 by applying a mask to the reconstructed transaxial images; post-filtering: a 3D low-pass post-filter was applied to reduce noise; 3D SPECT scans generated via Picker Prism Odyssey Rendering Software: outside (cortical) views: top 45% limits; interior views: top 8%, 15%, 45% limits.
The information provided by the SPECT scan, in combination with the patient’s clinical data, was used to decide upon the course of treatment or changes to an existing course of treatment. Treatment was predominantly psychopharmacological, but all patients received supportive psychotherapy and psychoeducation. The psychopharmacological modifications were guided by the functional abnormalities suggested by the brain SPECT scans. For example, if the anterior cingulate gyrus was over-perfused, then an SSRI could be added; or, for example, if a defect (under-perfusion) in an anterior temporal lobe was observed, then an anticonvulsant could be added. In a number of cases, the prefrontal cortex was hypoperfused, whereby a stimulant or a stimulating-antidepressant could be added as part of management. A summary of some frequently-observed findings on 3D SPECT brain scans and possible treatment strategies is presented in
Table 1.
3–6,15,16After stabilization (mean time: 17 months) of patients on the new treatment regime, a second brain 3D SPECT scan was completed. As mentioned above, GAF scores were obtained at the start of treatment and also after the second SPECT scan. Of the 30 patients, 2 patients did not want psychopharmacological treatment, leaving 28 patients who continued with scheduled treatment.
Twenty-eight control patients from the same community-based psychiatric clinic were retrospectively matched to the 28 patients who received SPECT scans and who completed scheduled treatment. Matching was done on the basis of initial GAF scores and diagnosis, and then, where possible, by sex and age. Control patients had not received any SPECT scans. Controls had received psychopharmacological treatment, as well as psychotherapy and psychoeducation. In
Table 2, the scanned patients are compared with the control patients.
SPECT Scan Image and Clinical Data Analysis
A quantitatively-based visual scoring system
17 for the 3D SPECT brain images was developed, and scans were scored by three community-based psychiatric practitioners without knowledge of patient clinical information. As some discussion occurred about the scans between these readers, scores were simply averaged, and interrater reliability was not computed. The clinician who provided care to the patients did not rate any of the 3D SPECT scans.
Since patients had heterogeneous findings on their scans (e.g., some with decreased perfusion in temporal lobes, some with decreased perfusion in prefrontal cortex, and so on), the visual scoring system
17 obtained a SPECT improvement score for each pair of before-and-after SPECT scans. The SPECT score was computed by giving 5 points for any major increase in areas with initially abnormally low perfusion (or major decreases in areas of initially abnormally elevated perfusion). For minor increases (or decreases) in perfusion relative to the initial scan, 1 or 2 points were assigned in each region. In the instance of markedly-increased perfusion, compared with an initial scan with globally decreased perfusion, a value of 10 points was assigned. The normal scans used as references were the age-adjusted normal 3D SPECT scan images provided by Amen.
6 Also used as an adjunct reference for normal scans was the work of Tanaka and colleagues,
14 which assessed patterns on 99m-Tc-ECD brain scans of “normal” adults (no psychiatric or neurological history), albeit 2D scans, and without regard to the areas of increased perfusion.
In the analysis of the results, if an improvement of ≥10 points occurred, then this was arbitrarily considered to be a positive improvement in the SPECT scan. If there was an improvement of <10 points, then this was considered to be no significant improvement (i.e., a negative result). Since the first part of this data analysis is to simply see whether there is a correlation between the SPECT scan score and the patient’s functioning (as shown by the GAF score changes below), and not the more clinically important question of whether SPECT scans result in better outcomes for patients, we decided against constructing a receiver operator characteristic (ROC) curve
18 and forcing a positive correlation by this or other preemptive statistical methods. The SPECT scan ratings would not influence patient outcome; they were simply there to see whether a basic correlation existed with patients’ functioning.
Half of a GAF scoring range (i.e., half of 10 points, or 5 points) was arbitrarily taken to indicate significant improvement in functioning. If the after-treatment GAF score exceeded the pretreatment GAF score by ≥5 points, then this was considered to be a positive GAF improvement. If there was less than a 5-point increase in GAF scores, then this was considered to be a negative GAF improvement. The relationship between patients’ clinical GAF scores and their SPECT scan scores was then analyzed by chi-square test.
With regard to the next objective of whether patients who underwent SPECT scans improved more in their functioning than matched case controls, as mentioned above, 28 control patients from the same community-based psychiatric clinic were retrospectively matched to the 28 patients who received SPECT scans and who completed scheduled treatment. Changes in GAF score (i.e., final GAF score – initial GAF score) of the patients receiving SPECT scans were compared with the changes in GAF scores of the matched controls. Paired t-test statistics were applied to these sets of GAF score changes.
Results
GAF improvement × SPECT improvement of the 30 patients who underwent pre-treatment and post-treatment SPECT scans are shown in the chi-square model presented in
Table 3.
The calculated chi-square value is 10.6, which allows rejection of the null hypothesis of no association of SPECT score changes with GAF score changes at a statistically significant value of p <0.01. Thus, an objective scoring of before-and-after treatment SPECT scans correlates with an objective scoring of patients’ functioning before and after treatment as shown by this chi-square analysis.
It is interesting to note, but not of statistical significance, that in this heterogeneous mix of community psychiatric patients, all 3 patients who showed a positive GAF score improvement but a negative SPECT improvement had been diagnosed with fibromyalgia. Work by Usui and colleagues
19 of performing ECD SPECT scans on 29 patients and 10 controls, similarly showed a wide variety of perfusion abnormalities in fibromyalgia patients.
Paired t-test statistics were then applied to the change in GAF scores of patients who underwent SPECT scans compared with the change in GAF scores of matched controls who did not undergo SPECT scans. The group of patients who received SPECT scans showed on average an increase of 13.8 in their final GAF scores, versus the matched-control patients, who showed on average an increase of 8.6 in their final GAF scores. The t value was 2.4, and the two-tailed p value equaled 0.023 (p <0.05). Thus, at the 5% significance level, patients who underwent SPECT scans improved more in their clinical functioning than matched case controls.
Discussion
Our retrospective analysis showed that before- and after-treatment SPECT scans correlated in a statistically significant manner with before- and after-treatment levels of patients’ functioning (GAF scores). On the important clinical level, our retrospective analysis showed that community patients who underwent 3D brain SPECT scans and SPECT-guided treatment improved more in functioning than their matched controls who did not undergo SPECT scans and SPECT-guided treatment. This latter analysis did not use any arbitrary scoring system other than the well-established GAF scores, and, in a very straightforward manner, compared outcome as indicated by GAF score improvement, of patients who had SPECT scans versus their matched controls who did not have any scans. Work by Amen and colleagues
20 has shown that the addition of brain SPECT modified the diagnosis or treatment in 79% of 109 consecutive psychiatric outpatients. In our study, the ability of SPECT scans to modify diagnosis or treatment of psychiatric outpatients was further extended to outcome.
Other reasons for the improvement in patients’ functioning should be considered. For the patient, there is the potential placebo effect of undergoing the actual brain SPECT scan, as well as the perceived psychological benefit of feeling that one is getting more attention from the clinician. Similarly, it is possible that the clinician felt more empowered to try different or more aggressive treatments in patients who received scans than in other patients in the practice.
SPECT is a well-established nuclear medical imaging technology. 3D SPECT combines the scanning data via thresholding functions to synthesize a 3D model of the brain. This retrospective study showed that 3D brain SPECT scanning improved community psychiatric patient outcome.
Acknowledgments
The authors thank Dr. Lisa Ehrlich for her assistance in making this study possible.
This work was approved by Canadian SHIELD Ethics Review Board #12-08-001.
No conflicts of interest disclosed by any author.