Behavioral variant frontotemporal dementia (bvFTD) is a common neurodegenerative dementia among those 65 years of age or younger.
1 The diagnosis depends on the presence of behavioral disinhibition, apathy/inertia, loss of sympathy/empathy, perseverative/compulsive behaviors, hyperorality, and a dysexecutive neuropsychological profile.
1 Perhaps most characteristic of patients with bvFTD is their degree of emotional blunting.
2 These patients have impairments in self-referential emotions such as embarrassment,
3 in the recognition of emotions from faces,
4 and in both cognitive and emotional aspects of empathy.
5In bvFTD, emotional blunting may be related to low emotional arousal as expressed in a low resting state of the autonomic nervous system. The neuropathology of bvFTD affects the prefrontal, anterior cingulate, insular, and amygdalar regions involved in maintaining emotions through the autonomic nervous system.
3 Studies have investigated the autonomic nervous system origin of emotional blunting in bvFTD but have focused on the phasic or physiological reactivity aspects rather than the tonic or resting aspects.
2,6 These studies often use an aversive stimulus such as an auditory startle to investigate autonomic nervous system reactivity.
7 Normally, after a defensive motor startle and a short latency, there is an autonomic nervous system orienting response that lasts approximately 3–10 seconds, with changes in heart rate, blood pressure, respiration, and skin conductance.
8,9 Prior research has observed acoustic startle responses among patients with frontotemporal lobar degeneration but has not focused on resting state measures.
3,10,11 However, the neuropathology of bvFTD suggests that tonic levels of autonomic nervous system may be primarily reduced.
This study assesses differences in skin conductance among patients with bvFTD compared with patients with Alzheimer’s disease and healthy control subjects. Of the major autonomic nervous system measures, skin conductance level (SCL) is the one that depends entirely on sympathetic tone. We use a paradigm that includes the introduction of an unwarned auditory startle stimulus to compare tonic SCLs with phasic skin conductance responses (SCRs) to aversive stimuli. On the basis of their clinical emotional blunting, we hypothesize that patients with bvFTD show low tonic SCLs despite the presence of SCRs. The results are discussed in terms of the implications for SCLs as a noninvasive measure for the differential diagnosis of bvFTD.
Discussion
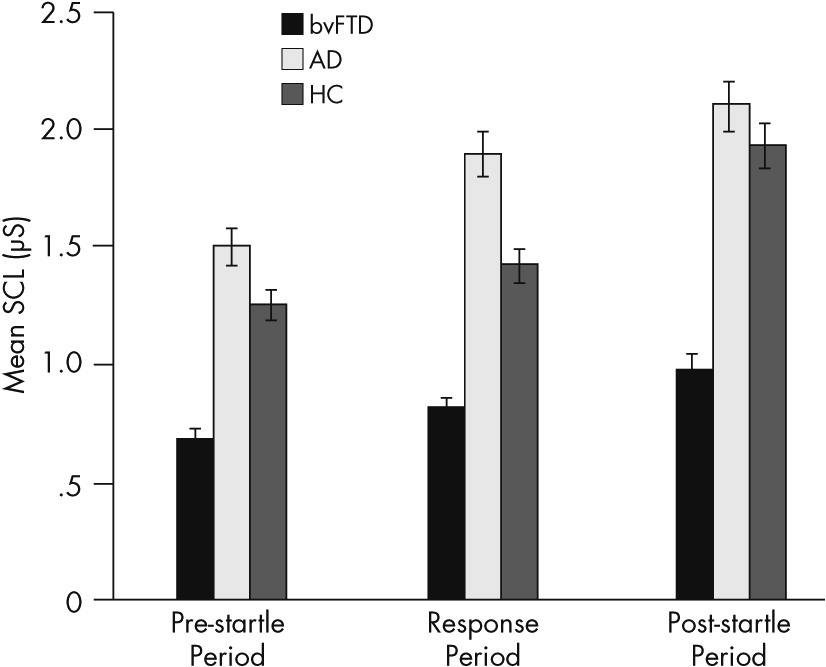
This study evaluates emotional blunting in bvFTD by examining their SC levels and responses. It confirms the presence of an orienting response to startle measured by SCRs in patients with bvFTD, similar to responses in patients with Alzheimer’s disease and in healthy control subjects. More significantly, this study documents lower SCLs throughout the prestartle and response periods among the patients with bvFTD compared with other groups. This indicates a low baseline sympathetic tone in bvFTD. In addition, scores on the Scale for Emotional Blunting negatively correlate with SCLs, particularly resting prestartle SCL. The implications of these findings are that patients with bvFTD have a low resting state of emotional arousal. Low SCLs may reflect some of the emotional blunting observed among patients with this disorder.
This is one of the few psychophysiological studies to compare patients with bvFTD with other patients with dementia such as Alzheimer’s disease.
3,11 The low SCLs among the patients with bvFTD clearly distinguish them from patients with the far more common Alzheimer’s disease. Distinguishing bvFTD from Alzheimer’s disease can be difficult, particularly if the Alzheimer’s disease is of early onset and accompanied by neuropsychiatric symptoms.
16 On pathology, approximately one in five patients diagnosed with bvFTD during life turn out to have the neuropathology of Alzheimer’s disease.
16 Consequently, a simple, low-technology, and relatively available noninvasive test, such as SC, can be helpful in distinguishing patients with bvFTD. Parenthetically, in the present study, the patients with Alzheimer’s disease show slightly higher SC measures than healthy control subjects, and, although not statistically significant, higher SC values could suggest decreased sensorimotor gating from entorhinal pathology in Alzheimer’s disease.
17Previous research has shown no differences in physiological reactivity and general somatic activity to an unwarned acoustic startle stimulus among patients with bvFTD compared with healthy controls.
3,10,11,18,19 These studies, which often included bvFTD as part of a larger frontotemporal lobar degeneration group,
3,11,18,19 reported decreases in embarrassability in frontotemporal lobar degeneration based on facial expression coding,
3 in recognition of sadness and fear in film clips,
11 and in emotional regulation.
18,19 These studies measured emotional regulation, not with simple SCLs, but with physiological composite scores.
3,10,11 The use of physiological composite scores may have conflated the initial defensive motor response to an aversive startle stimulus with the subsequent autonomic or orienting response.
Rather than composite scores, SC may be the best autonomic nervous system measure for resting sympathetic state and emotional arousal. Unlike heart rate or blood pressure measures, it is a pure cholinergic, sympathetic measure without confounding parasympathetic influences from emotional situations. SCL reflects the baseline state of the sympathetic autonomic nervous system and also reflects the degree of relaxation and emotional arousal.
20 In contrast, SCR is a transient change in SCL caused by a significant or novel stimulus and can markedly increase with anxiety, stressors, and emotional influences on the orienting reaction.
21,22 Some studies suggest that the higher the resting SCL, the greater the SCR amplitude and the lower the stimulus strength needed for an SCR.
8,23 However, SCL and SCR are dissociable and may reflect different brain mechanisms.
24 As seen in this study, event-related SCR occurred in bvFTD, whereas SCLs remained low.
Areas affected by bvFTD, including the prefrontal regions, insulae, and amygdalae, influence SC and emotional arousal.
15 Increased ventromedial prefrontal activity can decrease SC,
25 whereas increased orbitofrontal activity can either increase or decrease SCLs.
24 The insular cortex, particularly on the right, and the adjacent anterior cingulate cortex are also involved in regulating SC: both tonic SCLs and phasic SCRs.
26–28 The amygdalae further modulate SCRs, especially from fear and other emotional activity.
29 From these studies, it is probable that the structures most implicated in the low SCLs found in bvFTD in the present study are the anterior insular and the adjacent anterior cingulate cortices of the brain.
3,6 In sum, the underlying mechanism for low SCLs in bvFTD and increased emotional blunting may be attenuation of the resting sympathetic state and consequently low emotional arousal from disease in these areas of the brain.
To our knowledge, this is the first study to focus on SCLs in patients with bvFTD compared with patients with Alzheimer’s disease and healthy control subjects. Although bvFTD patients have a startle response, they have low SCLs compared with other groups. The findings indicate a low baseline sympathetic tone and suggest that a high threshold for reactivity contributes to the characteristic emotional blunting among patients with bvFTD. Furthermore, the measurement of SCLs could be useful as a simple, noninvasive test for bvFTD. Future research, using varying emotionally evocative stimuli, promises to clarify the value of this psychophysiological measure in the assessment of bvFTD.