Apolipoprotein E (ApoE) is a plasma lipoprotein that plays a critical role in the transport and metabolism of lipoproteins and cholesterol in the brain and other tissue.
1,2 ApoE has been implicated in numerous functions including neural development, regeneration, neuroprotection, and plasticity.
1,3 The human ApoE protein exists in three primary isoforms (E4, E3, and E2) that result in six genotypes: three homozygotes (E4/4, E3/3, E2/2) and three heterozygotes (E4/3, 4/2, E3/2).
4 Presence of the E4 allele, in particular, has been associated with neurotoxic effects and linked to various neuropsychiatric illnesses including Alzheimer’s disease,
5,6 depression,
7 and schizophrenia.
8Proteins consist of amino acids strung together in a sequence. Amino acids in proteins are called “amino acid residues” because they lose their water when combining to form the sequence. The sequence is generally reported (i.e., amino acid residues counted) from the N-terminal end containing the free amino group to the C-terminal end containing the free carboxyl group. The amino acid residue content of a protein is typically expressed as number of residues per mole of protein. The ApoE polymorphisms arise from single amino acid substitutions at residues 112 and 158, where ApoE3 has Cys-112 and Arg-158, ApoE4 has arginines at both sites, and ApoE2 has cysteines at these sites. These single amino acid polymorphisms have a profound effect on both the structure and function of the ApoE protein.
2,3 The resulting six ApoE genotypes (E4/4, E4/3, E4/2, E3/3, E3/2, E2/2) yield five groups with respect to the number of cysteine residues per mole (CysR/mole), as follows. ApoE4/4 has zero cysteine residues per mole (0 CysR/mole), E4/3 has one (1 CysR/mole), E4/2 and E3/3 each has two (2 CysR/mole), E3/2 has three (3 CysR/mole), and E2/2 has four (4 CysR/mole). The use of the number of CysR/mole to characterize the ApoE molecule converts the categorical ApoE genotype scale, consisting of the six distinct genotypes above, to a five-point continuous scale (0–4 CysR/mole). This allows the use of statistical analyses suitable for continuous variables (e.g., regression) to quantify the relations between various variables and ApoE.
ApoE and Posttraumatic Stress Disorder (PTSD)
Research evaluating the association between ApoE and PTSD is limited; however, several factors support a likely association. First, ApoE has been shown to play a key role in hypothalamic-pituitary-adrenal axis activity,
9 one of the primary pathways implicated in PTSD.
10 Second, ApoE has been associated with structural anomalies in the hippocampus and amygdala,
11–14 areas often implicated in PTSD.
15 Finally, ApoE is directly involved in neural functioning
16 and plasticity,
2,3 which have been shown to be aberrant in PTSD.
17,18Only one published study has examined the association of ApoE alleles and PTSD.
19 Given that cognitive impairments exhibited by individuals with PTSD are similar to those seen in Alzheimer’s disease (e.g., impaired memory, attentional deficits, and limited executive functions
20), Freeman et al.
19 hypothesized that the ApoE genotype, specifically the presence of ApoE4, might also relate to PTSD symptoms. Indeed, a relationship was found, although not as expected: impaired memory functioning and re-experiencing symptoms were associated with ApoE2 in the PTSD group rather than implicating ApoE4. Furthermore, ApoE4 was not associated with a risk to develop PTSD itself.
19 The results of this research, although demonstrating a link between ApoE and PTSD, are inconsistent with the authors’ predictions, as well as what would be hypothesized based on research on other neuropsychiatric disorders. Thus, additional research is needed to further understand the relationship between ApoE and PTSD.
Quantifying ApoE on the Basis of CysR/Mole
Consistent across research on the role of ApoE in neuropsychiatric illness, including PTSD, is the tendency to classify individuals based on the absence or presence of specific alleles (e.g., ApoE4
+/ApoE4
−), although little is known about how the different alleles lead to susceptibility to or protection from certain disease processes. For example, in Alzheimer’s disease research, the presence of at least one E4 allele is a risk factor,
21,22 except when in combination with the E2 allele, which is considered to be a protective factor.
23 Indeed, the risk associated with the E2/E4 genotype is generally considered normal risk—the same as that of the more common E3/E3 genotype. Closer examination into specific properties of the individual isoforms may shed more light onto the mechanisms behind associated vulnerabilities or their absence.
As mentioned above, the three most common ApoE isoforms, ApoE2, ApoE3, and ApoE4, can be distinguished based on cysteine-arginine interchanges at residues 112 and 158, with 2 CysR/mole present in ApoE2, 1 CysR/mole in ApoE3, and 0 CysR/mole in ApoE4.
2 This property provides an ordinal scale of classification
16 rather than categorizing individuals based on the presence or absence of specific isoforms. For example, an individual possessing the E2/E2 combination would have 4 CysR/mole; individuals possessing the E3/E2 combination would have 3 CysR/mole; individuals with either the E4/E2 or the E3/E3 combination would both possess 2 CysR/mole; and individuals with the E4/E4 combination would possess 0 CysR/mole. It is hypothesized that neurotoxic fragments associated with ApoE4 resulting from these minor sequencing changes affect the development of neuropathology and psychiatric disease.
2 In contrast, increased stability and decreased neurotoxicity associated with ApoE2 is likely due to the presence of cysteine molecules.
2 Thus, risk or protection associated with ApoE may be best examined using this biochemical scale based on CysR/mole rather than the presence of a specific isoform per se.
In the present research, we aimed to use the method above of expressing ApoE genotype in terms of CysR/mole to examine the association between ApoE and PTSD symptom severity. Although past research has implicated the role of ApoE2 in certain PTSD symptoms,
19 prior studies evaluating the association of ApoE and psychiatric disorders, as well as research suggesting neurotoxicity is associated with the relative absence of cysteines
2 (ApoE4>ApoE3>ApoE2), would not necessarily predict such a relationship. Instead, it is hypothesized that as the number of CysR/mole increases, PTSD symptom severity will decrease due to more structural stability and thus less toxicity in the brain.
Methods
Participants
Fifty-nine U.S. veterans participated in the study as paid volunteers. There were 53 male veterans (age: mean = 52.56 years, standard deviation = 14.43 years) and six female veterans (age: mean = 43.42 years, standard deviation = 8.00 years). The self-identified racial breakdown was as follows: one Asian, one more than one race, two African American, and 55 Caucasian. Veterans with a primary PTSD (N=49) or subthreshold PTSD (N=10) diagnosis according to medical chart were identified for recruitment. Veterans with active substance use disorders, serious chronic pain, and other central nervous system disorders were excluded from recruitment. Also excluded from recruitment were veterans with a history of psychosis or bipolar disorder and those with moderate or severe traumatic brain injury. Veterans who met eligibility criteria completed diagnostic interviews and self-report questionnaires and participated in a blood draw for genetic testing. The study protocol was approved by the Institutional Review Board at the Minneapolis VA Medical Center and participants provided written informed consent prior to the study.
Diagnostic Measures
The presence of PTSD was assessed using the Clinician-Administered PTSD Scale for DSM-IV-TR (CAPS)
24 or the Structured Clinical Interview for DSM-IV-TR (SCID)
25 PTSD module. For diagnostic purposes, CAPS symptom scores were converted to dichotomous scores using the SCID symptom calibration method.
26 The SCID symptom calibration method minimizes false positives and false negatives by providing empirically derived cutpoints for determining the presence or absence of PTSD symptoms. It is the preferred scoring method for the CAPS when differential diagnosis is the goal.
26 Subjects were diagnosed with subthreshold PTSD when impairment from reported symptoms was evident, but neither current nor lifetime symptom scores met SCID symptom calibration method diagnostic cutpoints for the diagnosis of PTSD.
In the present study, emotional responses other than fear, helplessness, or horror were accepted for criterion A2 when diagnosing PTSD and subthreshold PTSD, consistent with research demonstrating varying reactions to trauma.
27–29 A variety of traumatic events, including combat, childhood abuse, and sexual assault, were associated with PTSD and subthreshold PTSD diagnoses. Lifetime history of non-PTSD axis I diagnoses were evaluated with the SCID
25 using DSM-IV-TR criteria. Individuals with current non-PTSD diagnoses were excluded from analyses.
PTSD symptom severity was assessed using the PTSD Checklist (PCL-S).
30 The PCL-S includes 17 items assessing self-reported PTSD symptoms in relation to a stressful experience. Items are scored on a scale of 1 (not at all) to 5 (extremely), with total scores ranging from 17 to 85. Subscale scores are computed by totaling items pertaining to each of the three DSM-IV-TR criteria: B (re-experiencing), items 1–5; C (avoidant/numbing), items 6–12; and D (hyperarousal), items 13–17.
Lifetime trauma exposure was assessed with the Deployment Risk and Resilience Inventory.
31 In the present study, three Deployment Risk and Resilience Inventory scales measuring Predeployment Stressors, Combat Experiences, and Postdeployment stressors were included, for a total of 47 dichotomously scored items. A trauma score was computed by summing the items, and a corrected trauma score (items endorsed/items answered) was calculated to account for a small number of missing items. As a result, trauma scores ranged from 0 to 1.
Assessment of ApoE Genotype
DNA samples were genotyped using polymerase chain reaction (PCR) amplification followed by restriction enzyme digestion.
32 Each amplification reaction contained PCR buffer with 15 mmol/L MgCl
2 ng amounts of genomic DNA, 20 pmol ApoE forward (5N TAA GCT TGG CAC GGC TGT CCA AGG A 3N) and reverse (5T ATA AAT ATA AAA TAT AAA TAA CAG AAT TCG CCC CGG CCT GGT ACA C 3N) primers, 1.25 mmol/L of each deoxynucleotide triphosphate, 10% dimethylsulfoxide, and 0.25 µL Amplitaq DNA polymerase. Reaction conditions in a thermocycler included an initial denaturing period of 3 minutes at 95°C, 1 minute at 60°C, and 2 minutes at 72°C, followed by 32 cycles of 1 minute at 95°C, 1 minute at 60°C, and 2 minutes at 72°C, and a final extension of 1 minute at 95°C, 1 minute at 60°C, and 3 minutes at 72°C. PCR products were digested with
HhaI and separated on a 4% argorose gel that was stained with ethidium bromide. Known ApoE isoform standards were included in the analysis.
Data Analysis
Statistical analyses were carried out using the IBM SPSS statistical package (version 20; IBM, Armonk, N.Y.) and the Intel Fortran Compiler Professional edition (version 11.1). The ApoE genotype was recoded into a new variable to reflect the corresponding number of CysR/mole: E2/E2 = 4 CysR/mole (N=1); E3/E2 = 3 CysR/mole (N=5); E4/E2 = 2 CysR/mole (N=2); E3/E3 = 2 CysR/mole (N=33); E4/E3 = 1 CysR/mole (N=18); and E4/E4 = 0 CysR/mole (N=1).
Results
Effect of ApoE on PTSD Symptom Severity
The means and standard deviations of each of the PCL and trauma scores as a function of CysR/mole are presented in
Table 1. Overall, the PCL B score was positively dependent on the trauma score (p=0.015, t test on the slope, N=59, linear regression analysis). The regression equation was as follows: PCL B = PCL B score = 12.348 + 8483Trauma score.
Because we wanted to assess the relation between PCL B score itself and CysR/mole, we derived new PCL B values that were adjusted for this dependence by subtracting the effect of the trauma score: PCL B′ = PCL B Adjusted for Trauma = PCL B score − 8.483Trauma score.
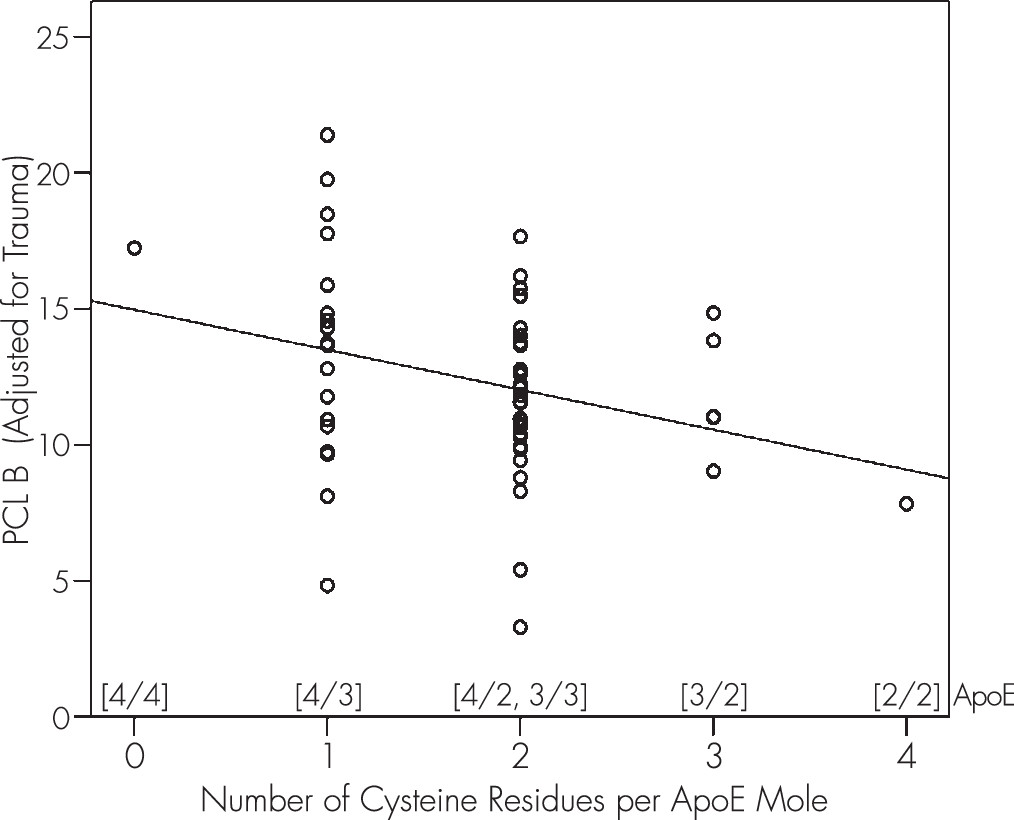
Then, we performed a linear regression analysis using PCL B′ as the dependent variable and CysR/mole as the independent variable (
Figure 1; p=0.022, t test on the slope, N=59, linear regression analysis): PCL B′ = 14.963 − 1.469 cysR/mole.
The negative slope in this equation means that there were fewer PCL B symptoms (adjusted for trauma) as the number of CysR/mole increased. A similar, although nonsignificant, negative trend between number of cysteine residues and PCL score was observed for both PCL C and PCL D symptoms; neither of these symptoms was significantly related to trauma.
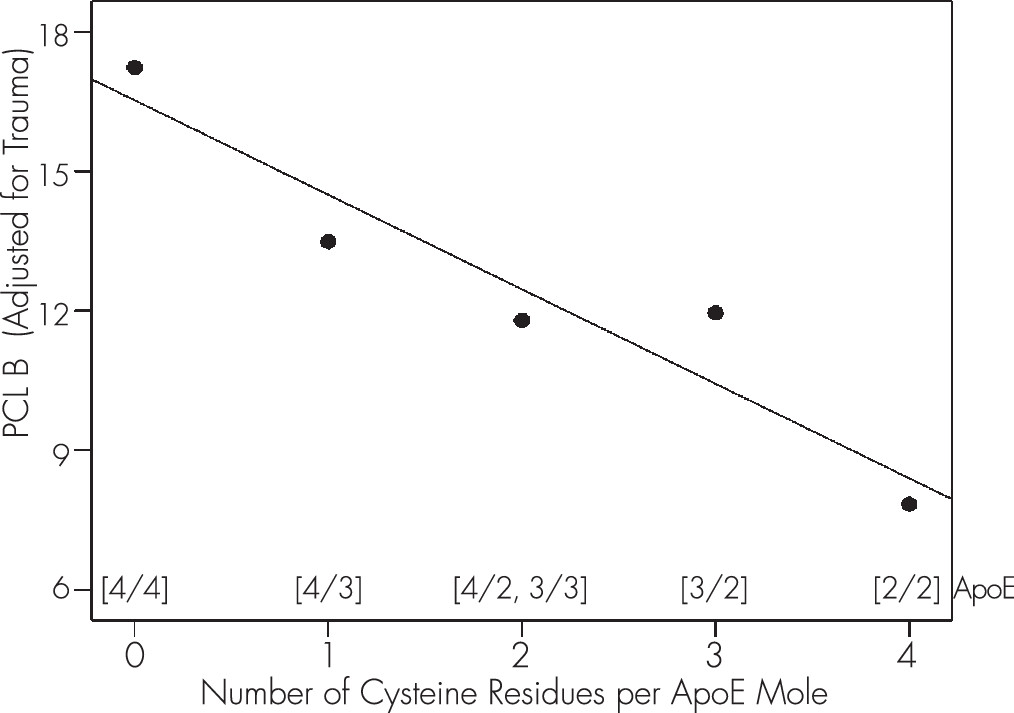
To further evaluate the relation between CysR/mole and PCL B′, we calculated the marginal means of PCL B′ and plotted those against CysR/mole. The effect, shown in
Figure 2, was highly significant (R
2 = 0.9, p=0.014, N=5).
Discussion
The present research evaluated the association between ApoE and PTSD symptom severity using a novel method for characterizing the molecular mechanisms of ApoE that distinguishes variants of the genotype based on the number of CysR/mole. The results support a significant negative association between the number of CysR/mole and PTSD re-experiencing symptoms, adjusted for trauma. That is, as the number of CysR/mole increased, there was less PTSD symptomatology. No significant associations were observed between CysR/mole and the remaining PCL scores, although the same negative trend was present.
Despite the significant association between the number of CysR/mole and PTSD re-experiencing symptoms found in the present study, there was significant variation in PCL re-experiencing scores within the different cysteine groups. This finding highlights individual differences in the association between ApoE genotype and PTSD symptom severity. Specifically, whereas certain cysteine groups may confer more or less risk for increased PTSD re-experiencing symptoms, additional risk or protective factors likely influence the severity of symptom presentation. For example, ApoE genotype may interact with various neural processes to moderate PTSD symptom severity. Indeed, a recent study evaluating the effect of ApoE on neural communication, which introduced the same ApoE characterization used here (i.e., based on the number of CysR/mole), found that neural properties differed significantly across the specific ApoE genotypes and varied in an orderly fashion with CysR/mole.
16 Future studies evaluating the combined effect of ApoE genotype and neural processes on PTSD symptom severity will be important for identifying factors that promote or prohibit healthy posttraumatic responses within at-risk ApoE genotypes.
The present study contributes to the growing interest in the genetic underpinnings of psychopathology and considers the effect of a gene that has been largely overlooked regarding its association with PTSD. In fact, only one prior study has examined the role of ApoE in PTSD. Freeman et al.
19 cited the presence of ApoE2 (2 CysR/mole) in the development of certain PTSD symptoms, including re-experiencing symptoms, whereas ApoE4 (0 CysR/mole) was not associated with PTSD symptoms. These findings are inconsistent with the authors’ predictions as well as other research linking ApoE4 to neuropathology and neuropsychiatric disorders. In contrast, the present research may have been successful in finding the predicted association between ApoE and PTSD symptoms due to its focus on the number of CysR/mole rather than the presence or absence of specific alleles. Consistent with that idea, Mahley and Huang
2 hypothesized that the relative impact of ApoE2 versus ApoE4 on disease is due to their respective number of CysR/mole. That is, the absence of CysR/mole in ApoE4 causes a propensity for structural instability and neurotoxicity, whereas the presence of CysR/mole in ApoE2 facilitates health. ApoE3, with 1 CysR/mole, falls in the middle of this continuum and is perhaps best thought of as neutral territory or with normal risk for disease.
Taken together, the current results suggest a protective function of ApoE CysR/mole—as opposed to the absence of ApoE4 per se—against the development of PTSD re-experiencing symptoms in the face of trauma. On the basis of recent research demonstrating a robust effect of ApoE on neural communication,
16 it is possible that greater symptom severity is a byproduct of blurred information exchanges associated with increased variability of synchronous neural interactions. Future research aimed at elucidating the underlying neural processes involved in this genetic influence on PTSD symptom severity is warranted.
Acknowledgments
Supported by the US Department of Veterans Affairs and the University of Minnesota American Legion Family Brain Sciences Chair.
The authors thank Kari Resel, Emily Van Kampen, Ann Marie Winskowski, Ryan Miller, Alina Shub, and Alexandra Alcorn for help in participant recruitment, consent, and data entry and Laura Kalipe for ApoE genotyping.