Mental imagery is a higher cognitive function, which is a mental representation of physical objects and events occurring in the absence of the appropriate stimuli. A specific type of mental imagery is mental rotation, described as the ability to imagine how a misoriented object would be appeared if rotated away from the presented orientation.
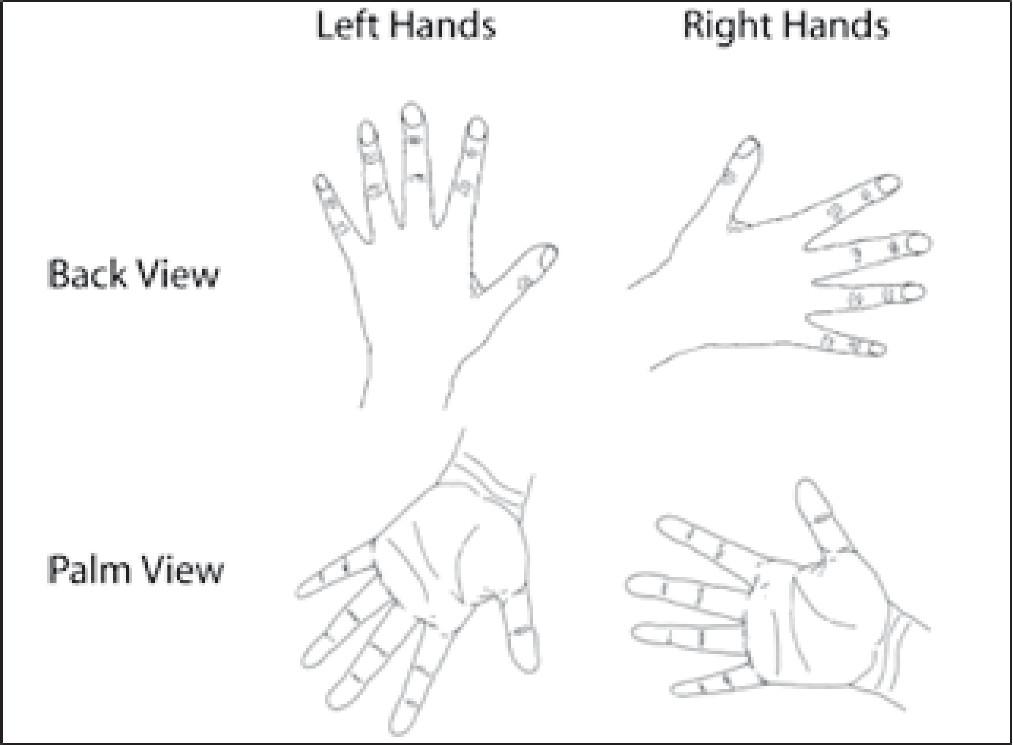
1 Mental rotation is typically studied by using Parson’s hand rotation paradigm in which individuals judge the laterality of pictures of left or right hands in different angles of rotation.
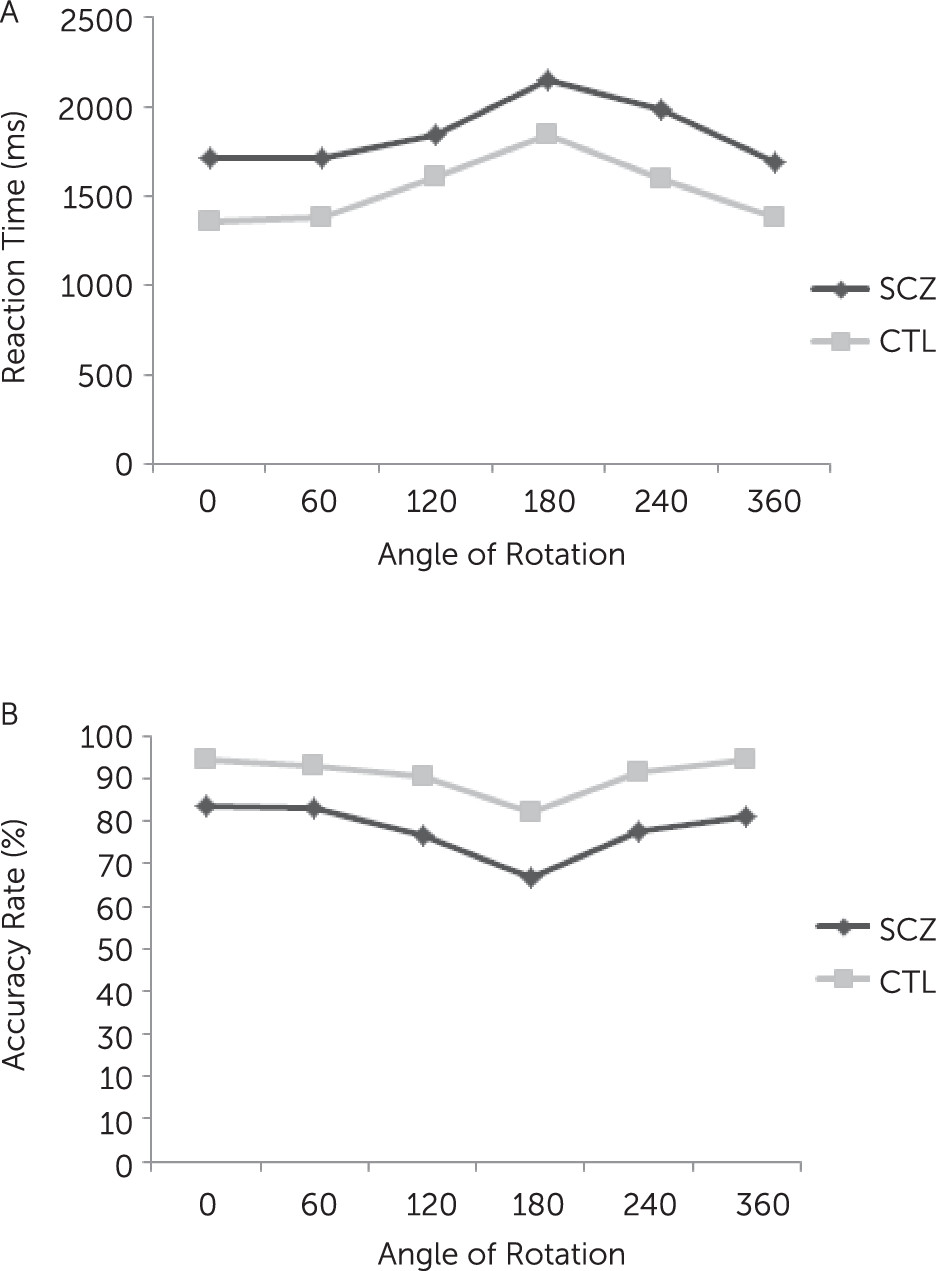
2 In general, reaction times and error rates increase as a function of the rotation angle of the stimulus, indicating that individuals engage in a cognitive process of mental rotation.
Recent studies indicated that mental imagery ability is compromised in individuals with schizophrenia.
3–6 For instance, studies have found that, although patients were slower and less accurate than control subjects on mental rotation tasks, they displayed the same decrease in speed and performance with increasing angles of rotation as control subjects.
4,7 Although this information suggests impaired mental rotation in the patients, there is a scarcity of evidence about electrophysiological correlates of the mental rotation process in patients with schizophrenia, which is the main aim of the present study.
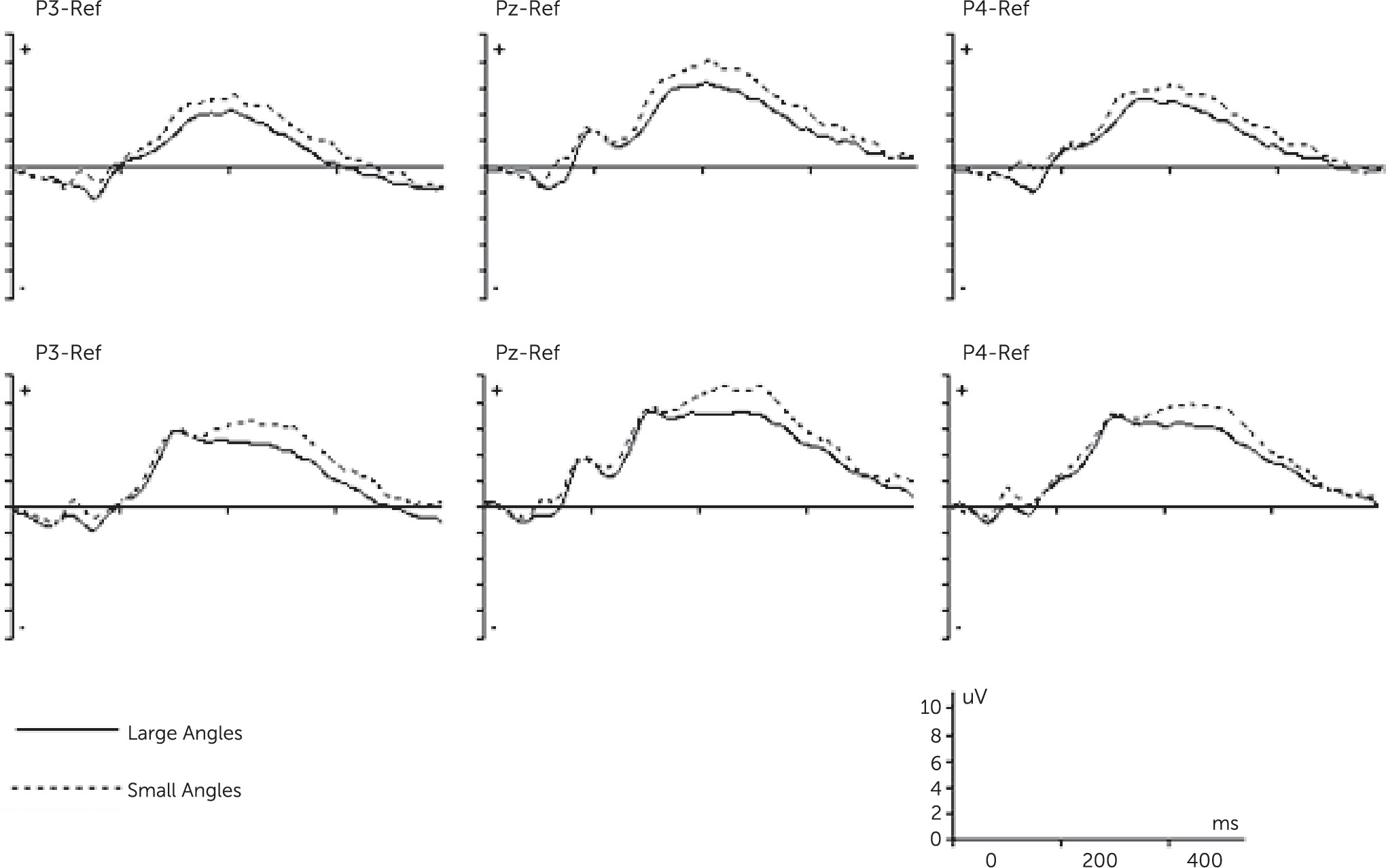
Many studies have examined mental rotation by means of event-related potentials (ERPs), because of its high temporal resolution. ERP studies have found the mental rotation process is reflected in a modulated positive wave of about 300–700 ms (P300), with decreasing amplitudes for increasing angles of rotation.
8,9 This slow decrease in amplitude is supposed to be due to a superimposed negativity on the concurrently prevailing P300 complex, so-called rotation-related negativity (RRN).
10–12 RRN is a parietal negative slow wave, most pronounced at the parietal electrodes, that is stronger for rotated compared with upright stimuli. RRN is assumed as an electrophysiological correlate of the mental rotation process.
9,13 Decreased amplitudes and increased latencies of RRN have been reported in patients with right hemiparetic cerebral palsy and patients with multiple sclerosis.
13,14 Although evidence indicates impaired mental rotation in schizophrenia, the question remains whether impaired mental imagery in patients with schizophrenia would be demonstrated by alteration of RRN in the mental hand rotation task.
In summary, the present study aimed to assess RRN during the performance of mental imagery in patients with schizophrenia. To reach this aim, the performance of a group of patients was compared with that of a group of healthy matched control subjects on a mental hand rotation task while simultaneously measuring ERP.
Discussion
This study aimed to investigate the neural correlates of impaired mental imagery during the performance of the hand rotation task. The main results can be presented as follows: at the behavioral level, patients performed hand rotation less accurately and more slowly than healthy participants. At the neural level, our results showed that compared with the control subjects, the patients with schizophrenia had less modulation of P3 amplitude (decreased RRN) during mental rotation.
The behavioral data indicated response times increased linearly with increasing angle of rotation of the stimuli for the both groups. This finding is in agreement with the original finding by Coopers and Shepard,
18 indicating the use of mental rotation strategy to perform the hand rotation task by our participants. Moreover, patients with schizophrenia responded relatively slower and had a higher error rate compared with control subjects, similar to findings of other studies.
4,7 These results suggest patients with schizophrenia may be characterized by a compromised process of mental representation of hands.
At the neural level, consistent with previous studies,
13,14 our results showed a significant general effect of increasing negativity with increasing angle of rotation (RRN) for both groups. Moreover, the observed decrease in RRN amplitude with increasing angle of rotation fits our behavioral data as they show an increase in response time as a function of angle. Accordingly, our findings further support the close relation between RRN and mental rotation process.
Our results showed that patients with schizophrenia had shallower trade-off function between angle of rotation and RRN amplitude relative to healthy participants, indicating an impaired mental imagery process at neural level. To our knowledge, this is the first study that extends previous behavioral studies in showing disruption of mental imagery at both behavioral and neural levels in patients with schizophrenia. Moreover, the pattern of P3 amplitude modulation in control subjects and the deficient P3 modulation in schizophrenia is consistent with two recent studies by Neuhaus et al.
19,20 Using the Attention Network Test, they showed impairment of patients with schizophrenias to modulate P3 amplitude, which was independent of duration of illness, age, and antipsychotic medications. These findings are extended in that we showed similar pattern of impaired P3 modulation in another task (hand rotation task) in patients with schizophrenia.
A possible explanation for RRN might be the general assumption that P3 amplitude is attenuated as task difficulty increases.
21,22 In our data, task difficulty increased when the orientation of hand stimuli increased from upright position, associated with smaller RRN. The less modulation of P3 in patients may indicate a failure to detect or to differentially process varying degree of task difficulty.
RRN is the standard ERP effect of mental rotation, and when superimposed on the P300 component, modulates its positivity at the parietal cortex. This modulation is associated to the degree of stimulus rotation and is suggested as a direct correlate of mental rotation.
9,23 Moreover, studies have shown that the posterior parietal cortex is activated during mental rotation, and RRN reflects the stronger activation of this area for stimuli with an increased rotation of angle.
9,24 Therefore, decreased RRN during mental rotation in the patients might indicate slightly less activation of posterior parietal cortex in the patients compared with control subjects, although it is activated in both groups during mental rotation.
These findings support the possible role of the posterior parietal cortex in the pathophysiology of patients with schizophrenia. Supporting evidence comes from studies suggesting that the dysfunction of the posterior parietal cortex is a putative candidate for many cognitive impairments in schizophrenia,
25,26 such as impaired spatial attention and oculomotor control, as well as deficits in motor control and motor imagery.
3,6,27In agreement with previous studies, we found less accuracy in response to the left hand stimuli than to the right hand stimuli in both groups.
13,14 This finding reflects the anatomical constrains of right-handed participants in the present study.
Finally, our results showed significant negative correlations between RRN amplitudes (P3 modulation) and SAPS total scores at P3 and Pz sites. As noted already, P3 modulation (RRN) reflects an electrophysiological correlate of the mental rotation process. A potential explanation for this finding might be that patients with positive symptoms have difficulties in monitoring the mental image of the rotation movement. In support, de Vignemont et al. found that hallucinatory patients were slower and less accurate than nonhallucinatory patients on mental rotation tasks.
4 They suggested that impaired mental rotation can reflect a disruption of action monitoring in schizophrenia, because the same brain areas are involved in both processes. Therefore, our findings may support the assumption of link between positive symptoms and disruption of action monitoring in schizophrenia. We did not find correlation between hallucination scores of SAPS and RRN, which might be due to our small sample size. Future research is required to repeat this finding and investigate the relationship between different subscales of SAPS and mental imagery in schizophrenia.
One of the limitations of this study is that the numbers of patients and control subjects were relatively small. Another limitation is the effects of antipsychotic and anticholinergic medications on performances of patients. However, no correlation was found between chlorpromazine equivalent dose and behavioral and ERP measures. Future studies are required to examine the association between RRN across a group of drug-naïve patients and in different subtypes of schizophrenia.
In conclusion, our findings indicate compromised underlying neural processing during mental imagery in schizophrenia. Specifically, although prior behavioral studies have provided only partial evidence for impaired mental imagery in patients with schizophrenia, this study provides better insight in the neural process underlying mental imagery in patients.