Mild traumatic brain injury (mTBI), which is often used interchangeably with the term “concussion,” accounts for 80%–90% of all head traumas.
1–5 The estimated annual occurrence of sports-related mTBI is 1.6–3.8 million,
6 and its incidence has been reported to increase at a rate of 16.5% per year.
7,8 The most common complaints that are reported after mild to moderate head injuries include headache, dizziness, fatigue, irritability, insomnia, loss of consciousness, and memory loss.
9 Postconcussive symptoms usually resolve over a period of several days
10; however, a subgroup of patients experience continuous postconcussive difficulties and neuropsychological deficits that can persist for months or even years.
11–28The diagnosis of mTBI is typically based on subjective clinical measures (i.e., symptom checklists).
29 However, these measures lack objectivity, and decisions based upon them are questionable. For example, in the case of athletes, premature return to play may jeopardizing the individual’s career, whereas in the case of potential secondary gains, symptom exaggeration or simulation may go unnoticed. Thus, typical assessment procedures have been criticized for bearing the risk of misclassification,
30,31 and calls for more reliable forms of diagnosis have been made. Because research on concussion has revealed diverse chronic alterations of brain function affecting the cognitive,
32–35 affective,
36 and motor domains,
19,37,38 regardless of time since injury,
39 the goal of the present study was to advance diagnostic methods by analyzing brain oxygenation patterns in subjects suffering from the long-term effects of mTBI.
The majority of patients with mTBI usually show normal structural CT and MRI scans when results are inspected visually; Belanger et al.
40 therefore suggested that future research should focus on finding functional abnormalities by using alternative neuroimaging techniques. This suggestion is supported by evidence showing that individuals who sustain mTBI often exhibit abnormal neuropsychological profiles, such as deficits in working memory, attention, information processing speed, and, more generally, in executive function, which has been linked to frontal lobe function.
12,41–45 Indeed, data from resting metabolic measurements using positron emission tomography (PET) and single-photon emission computerized tomography have indicated that frontal cerebral blood flow decreases after traumatic brain injury (TBI).
46–49 Of further relevance to the present study, hypometabolism in frontal and temporal brain regions with normal neuroanatomical scans
50–52 have been correlated with deficient neuropsychological performance and postconcussive symptoms.
39,52 In a comparison of symptomatic concussed athletes with normal controls performing working memory tasks, Chen et al.
17 observed the expected frontocortical activations in the normal controls but not the concussed athletes. The differences in the brain regions involved concerned the mid-dorsolateral prefrontal cortex (DLPFC) that was bilaterally activated in symptom-free subjects with or without a history of mTBI but less so in symptomatic subjects who also showed poor performances of working memory tasks. In further studies, Chen et al.
36,53,54 observed that all concussed athletes had normal structural imaging results, affirming the necessity of functional brain activation studies.
In contrast to these findings, hypermetabolism of these regions in patients suffering from postconcussive symptoms has also been documented.
8,55–57 McAllister et al.
8,55,56 showed enhanced and a more widespread BOLD signal (blood-oxygenation-level-dependent) in concussed subjects performing a series of cognitive tasks. Specifically, higher working memory processing loads were associated with increased activation in the DLPFC in concussed subjects who successfully performed an “n-back” working memory task. However, mTBI patients showed disproportionately increased activation during a moderate processing load condition.
56 Jantzen
57 also showed increased activation in parietal, lateral frontal, and cerebellar regions in concussed subjects when compared with preinjury and the absence of changes in cognitive performance. Thus, it has been argued that a hyperactivation of the prefrontal cortex and possibly other regions of interest in mTBI may represent a “neural inefficiency”
58 that has been linked to diminished performance of cognitive tasks in neurological patients.
59–62 However, n-back tasks did not demonstrate convergent validity with established working memory measures.
63 We therefore addressed this controversy by investigating whether a mTBI is accompanied by increased or decreased frontal cerebral blood flow during a working memory task using functional near-infrared spectroscopy (fNIRS).
Although functional MRI (fMRI) seems to be a valuable tool for assessing brain activation and information regarding clinical decisions about concussion,
64 it is practical only in large clinics and research settings. The technology requires expensive equipment, technical personnel, and space. In contrast to fMRI, fNIRS offers a practical, portable, and relatively inexpensive alternative to assess correlates of brain oxygenation with the option to coregister other neurophysiological and behavioral data in a “near natural” environment.
65–67 However, fNIRS is largely unknown to clinical neurology.
68 Although fNIRS reliably detects changes in cerebral hemodynamics after severe TBI,
69 only one study has reported preliminary fNIRS results in mTBI.
70 Kontos et al.
70 reported decreased activation patterns in frontal brain regions for concussed patients during several memory tasks when compared with healthy control subjects; however, they communicated their results as preliminary, because the study concerned only a small number of participants (nine symptomatic patients and five age-matched healthy controls).
The present study investigated the hemodynamic response of frontal brain regions from both hemispheres of concussed patients with severe postconcussive symptoms in comparison with concussed subjects with minor symptomatology and healthy controls. Because previous controversies might have been the result of different experimental designs,
70 we employed the same working memory tasks used by Chen et al.
17 Thus, we hypothesized that concussed subjects who suffer from severe postconcussive symptoms show decreased functional brain oxygenation patterns compared with concussed participants with minor symptomatology and healthy controls.
Methods
Subjects
Thirty subjects were recruited by means of a billboard notice at the German Sport University Cologne (Cologne, Germany). Only right-handed individuals were selected for the study. Handedness was established using two questionnaires, the Edinburgh Inventory
71 and a questionnaire currently used at the Montreal Neurological Institute (MNI).
72 All subjects provided written informed consent to participate in the study.
Each subject underwent a neurological and psychiatric-psychosomatic examination. A psychiatrist who was also a trained neurologist and specialist in psychosomatic medicine and psychotherapy conducted all clinical examinations. Five subjects were excluded from the study because their symptoms were related to somatoform and dissociative disorders (according to ICD-10 codes F44 and F45) and could not be differentiated from permanent postconcussive difficulties.
Thus, 17 individuals (mean age = 27.1, SD = 11; 10 women, 7 men) who had sustained mTBI, and eight normal control subjects matched in age (mean age = 26.5, SD = 9; 6 women, 2 men) participated in the study. The study was approved by the local ethics committee of German Sport University Cologne.
Neuropsychological and Symptom Complaint Measures
Participants were questioned by the clinical examiners regarding the occurrence of mTBI, the presence or absence of loss of consciousness, and the presence or absence of postconcussive symptoms according to the Concussion Symptom Inventory (CSI).
73 Subjects with a history of concussion were classified into one of two groups: severe or mild postconcussive symptomatology. This classification resulted in the following three groups of interest: concussed subjects with severe postconcussive symptoms, concussed subjects with minor postconcussive symptoms, and a normal control group without a history of a mTBI. Descriptive statistics of the three groups are summarized in
Table 1. Individual information about the concussed patients with postconcussive symptoms is given in
Table 2. Given the presumed association between mTBI and depressive symptoms,
36,74 the Beck depression inventory (BDI) was used in addition to the clinical examination to control for symptoms of depression.
We then used the Sport Concussion Assessment Tool 2 (SCAT2), which has been endorsed for sideline assessment and clinical use.
75,76 SCAT2 incorporates a symptom evaluation scale, the Glasgow Coma Scale,
77 the Standardized Assessment of Concussion (SAC),
78 a modified version of the Balance Error Scoring System (BESS),
79 and the Maddocks questions.
80 These scales are combined to yield a total SCAT2 score.
The symptom evaluation for postconcussive symptoms implemented in the SCAT2 scale is based on an individual’s present state. Participants are asked about 22 different symptoms, such as headache, pressure in the head, dizziness, sensitivity to light, irritability, and so forth. The number of symptoms are combined into a postconcussive symptom (PCS) score with a maximum score of 22. Each symptom is further graded from 0 to 6, with 0 indicating no symptoms and 6 indicating severe symptoms. A symptom severity score is then calculated with a maximum score of 132 (i.e., 22 × 6).
The Glasgow Coma Scale provides a standardized method for grading the severity of TBI and its resulting symptoms.
77 It is composed of three parameters: eye response, verbal response, and motor response. It is scored between 3 and 15, 3 being the worst, and 15 the best. A GCS Score of 13 or higher correlates with mTBI.
The Standardized Assessment of Concussion was developed as a brief measure of neurocognitive functioning to evaluate the immediate effects of mTBI.
78 It includes five orientation questions, a five-word list-learning test, digits backward, reversing the months of the year, and a delayed recall of the word list. Summing scores from all these tasks yields a 30-point composite score. It has been used to detect cognitive abnormalities after mTBI in several studies.
10,81,82The BESS test is a standardized sideline instrument.
79 It requires that the subject maintains three different stances (double, single, and tandem) for 20 seconds per stance. Each stance must be performed both on a firm surface and on a piece of 10-cm-thick foam. While the subject is in each stance, the examiner must identify six types of errors: 1) lifting hands off of the iliac crest; 2) opening the eyes; 3) stepping, stumbling, or falling; 4) moving the hip into more than 30° abduction; 5) lifting the forefoot or heel; and 6) remaining out of the test position for more than 5 seconds. Scoring is based on the total number of observed errors. Several investigations have demonstrated the reliability of this scoring system.
83,84In order to test an athlete’s orientation in time and space, the five Maddocks questions are incorporated into the SCAT2 assessment (e.g., “At what venue are we today?” and “Did your team win the last game?”). The false positive rate varies between 29% and 68%.
80 Therefore, recent mTBI guidelines recommend using the Maddocks questions in combination with other diagnostic tools.
75,85,86Finally, the SCAT2 score represents an overall score of all previous tests. Baseline values for the SCAT2 have been established in healthy and concussed subjects.
87–89 Valovich et al.
88 reported an average SCAT2 score of 88.3 ± 6.8 for healthy adolescent athletes. Similar results have been reported by Jinguji et al.
87 (i.e., an average SCAT2 score of 89 ± 6 for healthy high school athletes without a history of a mTBI). The average SCAT2 score of concussed subjects was 83.8 ± 7.7 in the study of Virji-Babul et al.
89Task Description
We used working memory tasks devised by Dr. Michael Petrides that have been validated in neuropsychological studies of patients with lateral frontal lesions,
90 functional neuroimaging work with PET,
91 and fMRI.
92 These tasks have been used previously in fMRI investigations of functional abnormalities in concussed athletes.
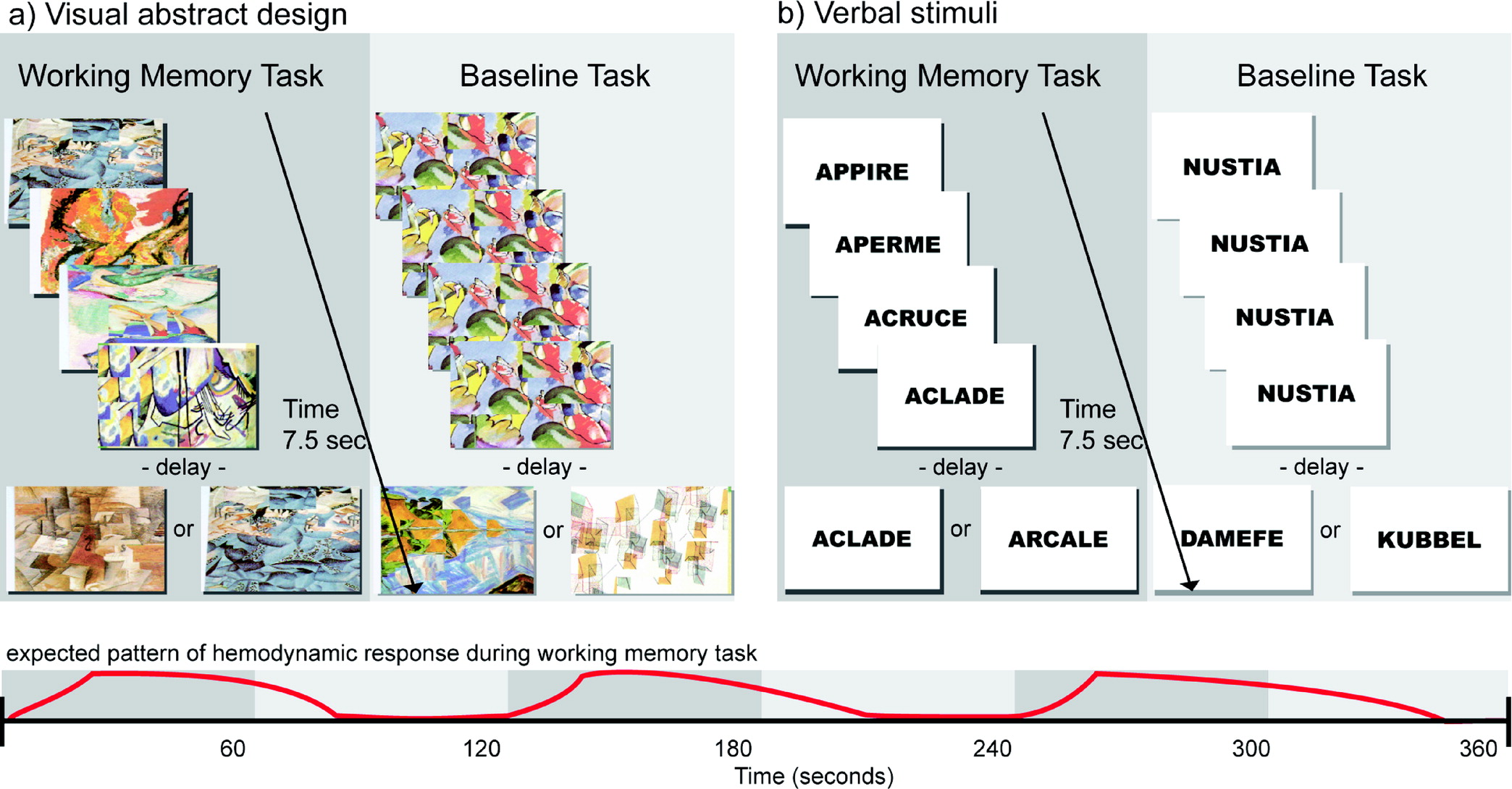
17,54,59 The tasks consist of two subsections: verbal stimuli and abstract visual stimuli. Subjects were familiarized with a set of five items to be used throughout both versions the test (five abstract drawings for the visual version and five abstract words for the verbal version). During each trial, four out of these five items were presented successively in random order at the center of a computer screen, and the subject had to identify the one item from the set that was not presented (
Figure 1). After the presentation of the fourth item, a delay of 1 second was introduced. Immediately after this delay, a test item was presented, and the subject had to indicate whether this test item was one of the four items presented before the delay or the fifth item that had not been presented. The subjects indicated their responses by pressing a mouse button (right button = yes, left button = no). Each subject had 1.5 seconds to respond, after which a new trial began.
In the baseline control condition, the format and type of stimulus presentation, mode of response (i.e., pressing the left or right mouse button), and timing of events were identical to those of the experimental working memory conditions. The stimuli used in the baseline condition were unrelated to those used in the verbal and visual design working memory conditions. The control condition was introduced to obtain baseline activation to “subtract out” any activation related to the motor and perceptual components of the working memory task. During the stimulus presentation in each trial, a single item was presented four times in succession at the center of the screen, followed by a delay of 1 second. After the delay, one of two items associated with either the left or right mouse button was presented at the center of the screen, and each subject had 1.5 second to respond. Before each trial, the subjects were told which one of the two items was associated with the left mouse button and which one was associated with the right mouse button. Thus, in the baseline control condition, each subject was responding in a fashion identical to the working memory experimental conditions, but these motor responses were based on conditional associations learned before the trial rather than on working memory. Each subject practiced the working memory task extensively before the beginning of the fNIRS session so that the sets of target items were familiar.
fNIRS Acquisition and Analysis
Cerebral oxygenation changes were recorded using a near-infrared optical tomographic imaging device (DYNOT Imaging System, NIRx; wavelengths = 760 nm, 830 nm; sampling rate = 6.88 Hz). The methodology and underlying physiology are explained in detail elsewhere.
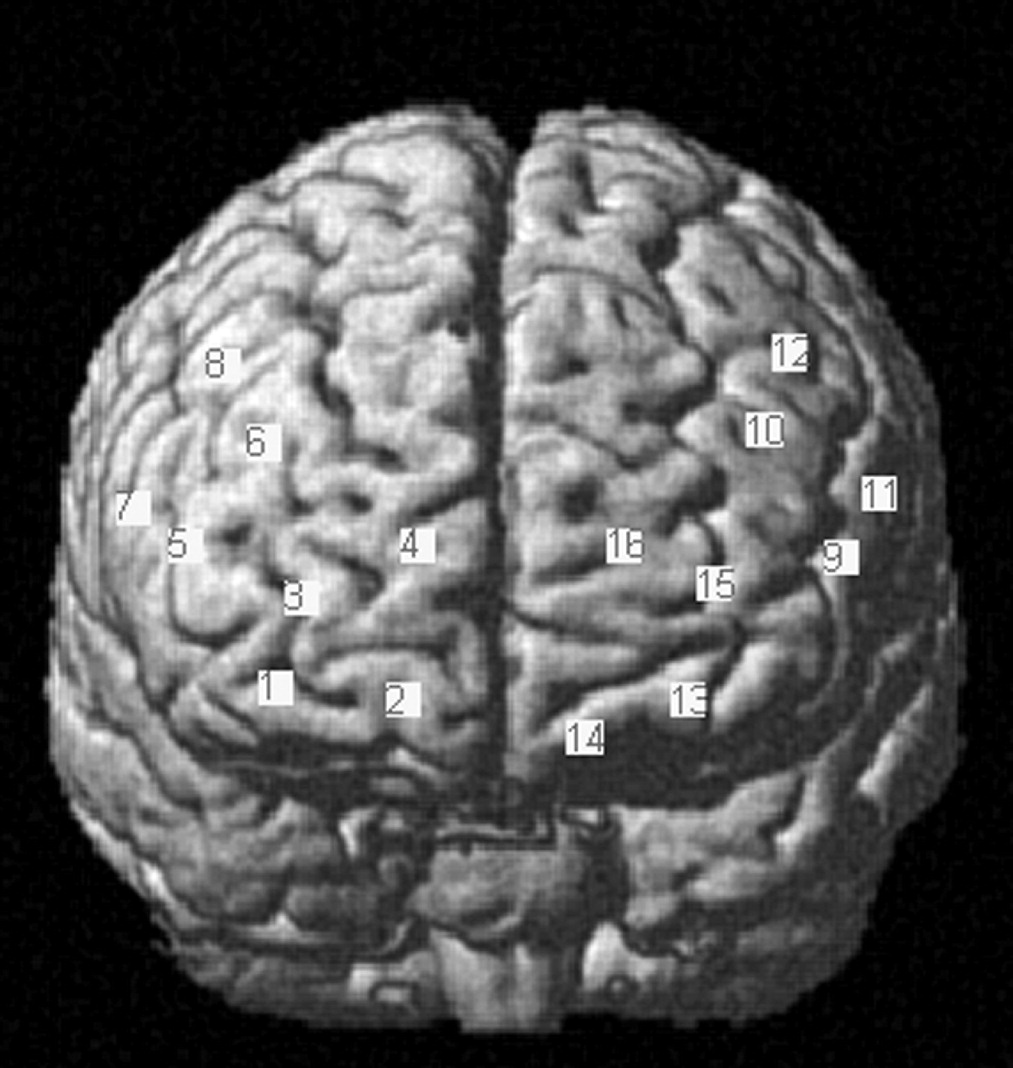
93,94 A total of 20 optodes (four emitters, 16 detectors) were placed in four 1 × 4 grids above the frontal cortex of each hemisphere, resulting in 16 channels of measurement (
Figure 2). Optodes were placed above the frontal lobes symmetrically and bilaterally at an interoptode distance of 3 cm according to the 10–20 system
95 . Coordinates of optode positions were collected for spatial registration of fNIRS channels into the standard brain from the MNI space according to Singh et al.
96 using a three-dimensional digitizing system (Zebris 3D Measuring Systems, Zebris Medical GmbH). Coordinates of MNI and respective Brodmann areas of each channel were exported using the NIRS-SPM toolbox.
97,98 Channel positions covered identical regions above both hemispheres, including the frontopolar area, orbitofrontal area, dorsolateral prefrontal cortex, inferior prefrontal gyrus, pars triangularis (Broca’s area [BA]), pars opercularis (BA), and frontal eye fields (
Figure 2). Optodes were mounted with a customized plastic hard shell system on the subject’s head to gain placement stability and to avoid movement artifacts.
Data were analyzed using the MATLAB-based Homer2 software package from the Optics Division of the Athinoula A. Martinos Center for Biomedical Imaging.
99 Sixteen channels were converted to hemoglobin concentration changes according to Cope et al.
94 The raw intensity data were normalized to provide a relative (percent) change by dividing by the mean of the data. Because fNIRS data can be affected by movement artifacts, each individual channel for each subject was visually inspected, and movement artifacts were corrected using the artifact correction algorithm developed by Scholkmann et al.
100 High-frequency components, mainly caused by the heartbeat, were attenuated by a low-pass filter at 0.1 Hz. To correct drifts and slow fluctuations, an additional high-pass filter at 0.001 Hz was applied. In order to obtain an average response to stimulation, the data were block averaged from 2 seconds before stimulus onset to 60 seconds after onset. Statistical analyses focused on the increases in oxygenated hemoglobin (HbO
2), because these appear to reflect task-related cortical activation more directly than decreases in deoxygenated hemoglobin (HHb), as evidenced by the stronger correlation between the former and the blood-oxygenation-level-dependent signal measured by fMRI
101 and by the results of animal studies.
102 To account for the influence of sensory stimulation when executing the computer mouse for the manual responses, we subtracted the block averages of the baseline condition from the memory task condition. Statistical significance of the factors “task” (verbal stimuli or visual abstract designs) and “group” was then analyzed by analysis of variance (ANOVA) with repeated measurements using SPSS (IBM SPSS Statistics, version 22) for each channel. Multiple post hoc pairwise comparisons were corrected using Bonferroni corrections. To adjust the multiple testing, correction for controlling type I errors by the false discovery rate was applied (data available from Benjamini and Hochberg, 1995; Singh and Dan, 2006).
Results
Group Specifics and Postconcussive Symptom Assessment
All subjects had a score of 15 on the Glasgow Coma Scale, a score of 5 for the Maddocks questions, and a BDI score ≤10. All brain injuries were therefore classified as mild. No significant differences between groups were found regarding the BDI score [F(2,24) = 2.835, p=0.08], or their age [F(2,24) = 0.287, p=0.75]. Furthermore, the two concussed groups did not differ regarding the number of months since the concussion [t(15) = −0.255, p=0.80].
Significant differences between groups were found for the number of symptoms (i.e., the CSI score [F(2,24) = 21.609, p<0.001]) and the PCS score [F(2,24) = 36.407, p<0.001]. Groups further differed significantly in the number of balancing errors on the BESS [F(2,24) = 4.112, p<0.05], the SAC [F(2,24) = 6.018, p<0.01], and the total SCAT2 score [F(2,24) = 34.585, p<0.001]. Post hoc comparisons showed that the concussed group with severe symptoms differed significantly from the concussed group with minor symptoms (p<0.001) and the control group (p<0.001) because of their increased quantity of postconcussive symptoms (CSI and PCS scale). Furthermore, the concussed group with minor symptoms showed significantly more postconcussive symptoms than the control group within the PCS scale (p<0.05). Post hoc comparisons of the balance scores showed significantly more balancing errors for the concussed group with symptoms when compared with the control group (p<0.05). Regarding the SAC score, post hoc tests revealed significantly lower scores for the concussed group with symptoms than the control group (p<0.05) and the concussed group with minor symptoms (p<0.05). Post hoc comparisons further revealed that the concussed group with symptoms differed significantly from the concussed group with minor symptoms (p<0.001) and the control group (p<0.001) because of a lower total SCAT2 score (
Table 1).
Behavioral Analysis – Working Memory Task
ANOVA with repeated measurements on the percentage scores of correct answers revealed a significant effect for group [F(2,22) = 4.073, p<0.05]. Post hoc comparisons revealed that the mean performance of the concussed group with severe symptoms was significantly lower than the performance scores of the control group (p<0.05). The same analysis indicated that the overall group performances were significantly different for the verbal and visual design versions of the task [F(1,22) = 7.754, p<0.05]. Post hoc comparisons revealed that the average score of correct answers was higher during the memory task with the visual stimuli compared with the verbal stimuli (p<0.05).
fNIRS Results
Effect of group
Calculating an ANOVA with repeated measurements on the ∆HbO
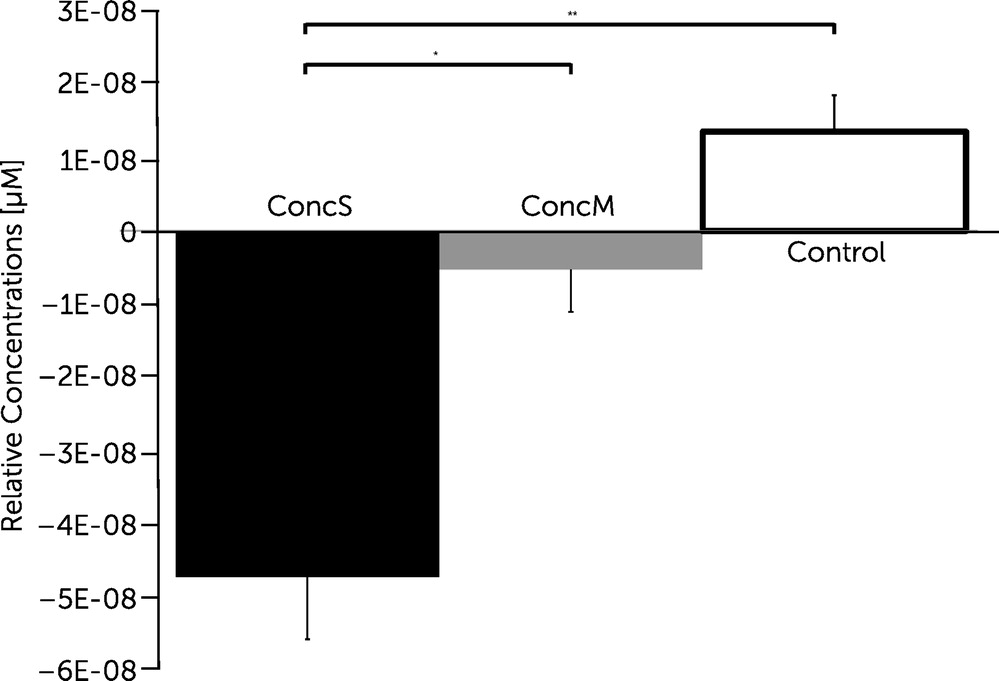
2 values revalued for the factor “group” revealed significant differences in four channels (channels 3, 4, 5, and 7) covering regions of the frontopolar area (BA 10), orbitofrontal area (BA 11), dorsolateral prefrontal cortex (BA 46), and pars triangularis (BA 45) of the right hemisphere [channel 3, F(2, 22) = 6.286, p<0.01; channel 4, F(2, 22) = 6.219, p<0.01; channel 5, F(2, 22) = 5.092, p<0.05; channel 7, F(2, 22) = 4.815, p<0.05] and in two channels (channel 11, 12) covering regions of the pars triangularis (BA 45), pars opercularis (BA 44), and dorsolateral prefrontal cortex (BA 9/46) of the left hemisphere [channel 11, F(2, 22) = 6.448, p<0.01; channel 12, F(2, 22) = 8.276, p<0.01]. Post hoc comparisons revealed significantly decreased changes in oxygenation for the concussed group with symptoms compared with the control group within the latter channels (channels 3, 4, 5, 7, 11, and 12;
Figures 3 and
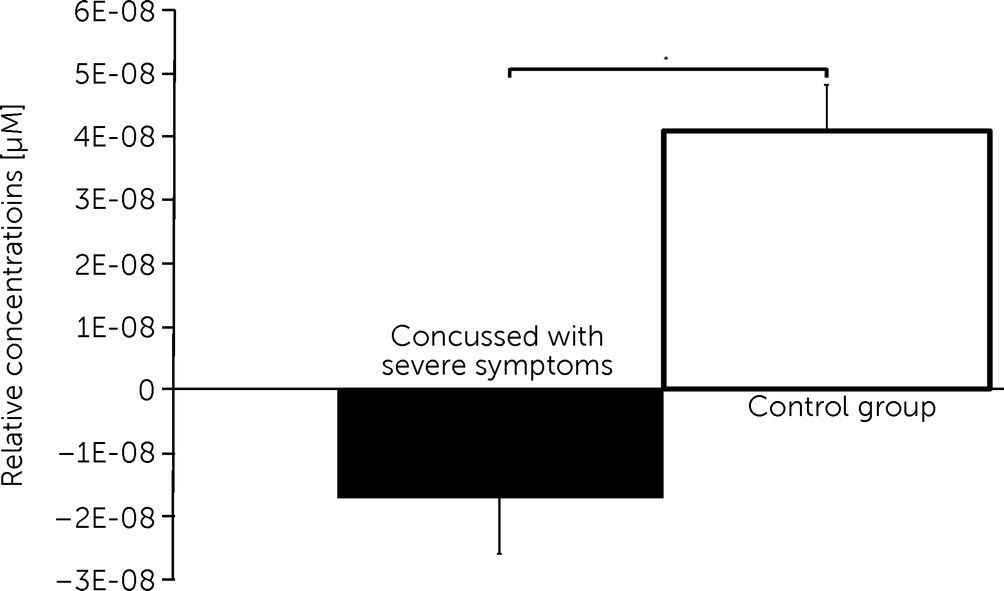
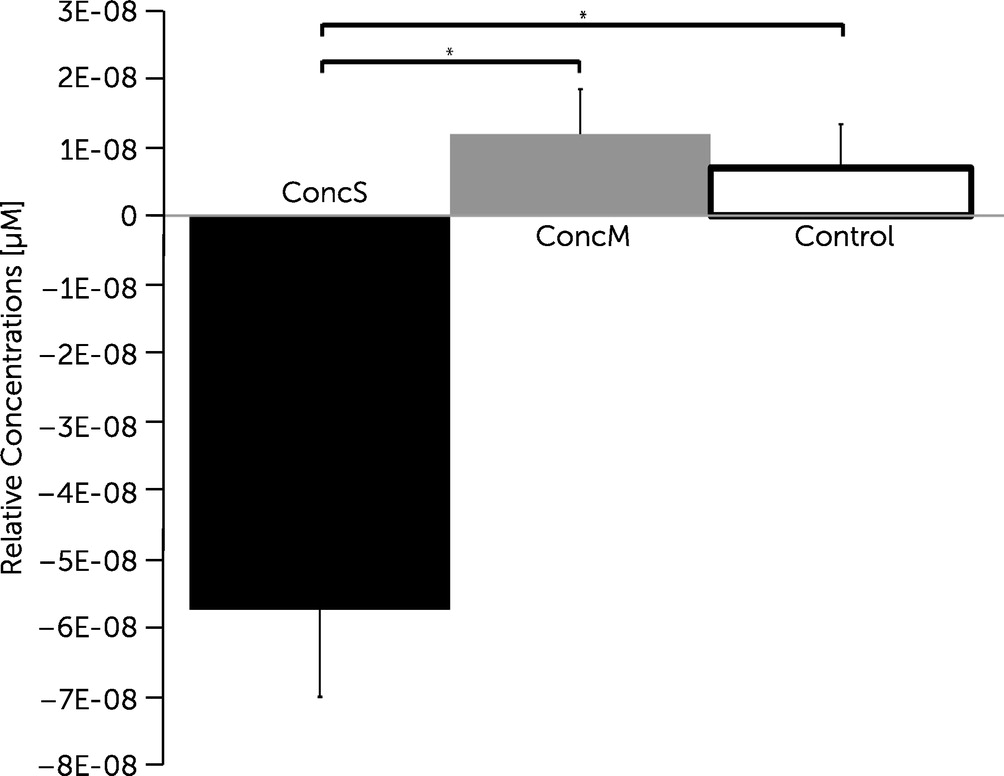
4). Post hoc comparisons further revealed significantly decreased changes in oxygenation within channel 12 covering the DLPFC for the concussed group with symptoms when compared with the concussed group with minor symptoms (
Figure 5).
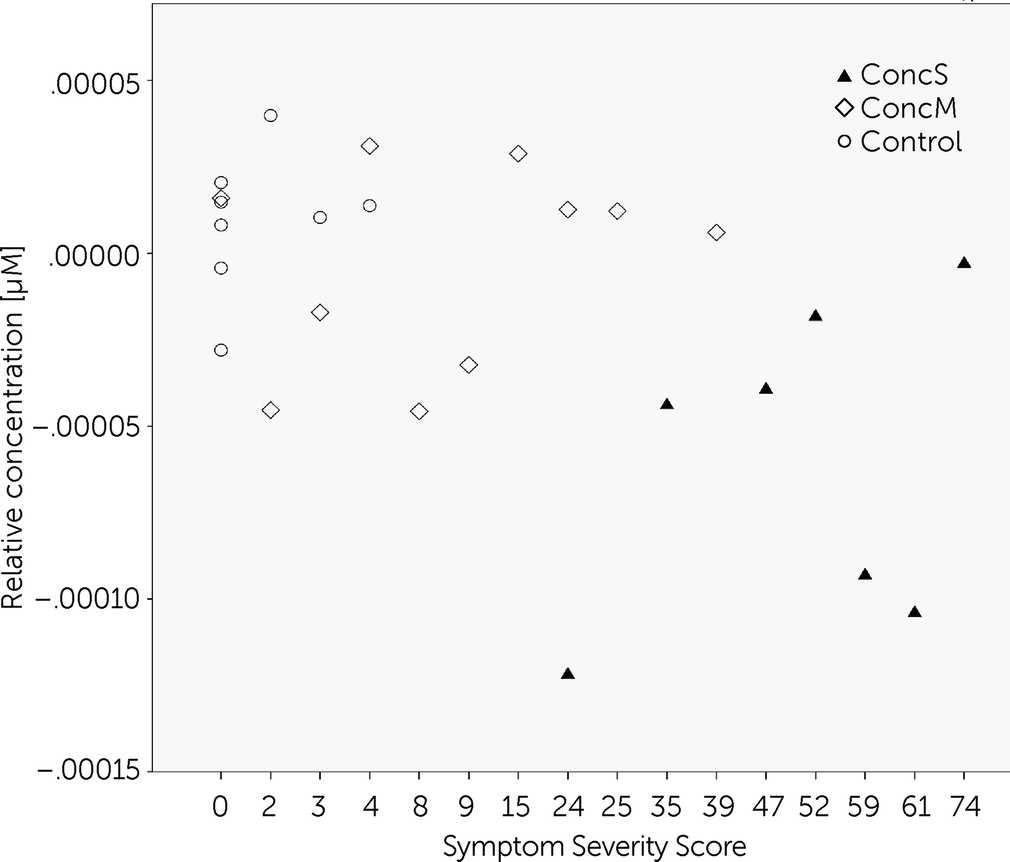
Correlation analysis
In order to reveal the relationship between the severity of symptoms and oxygenation changes, we performed Spearman rank-order correlation tests within channel 12 (dorsolateral prefrontal cortex [BA 9/46] of the left hemisphere). This channel showed significant differences between groups, in particular between concussed groups with PCS and with minor symptoms. A significant negative correlation was found for the symptom severity score and brain oxygenation during the visual memory task (r
(25) = −0.443, p<0.05;
Figure 6).
Interaction effect of “group” and “task”
The interaction of “group” and “task” reached significance in channel 8, covering the dorsolateral prefrontal cortex of the right hemisphere (BA9/46) [channel 8, F(2, 22) = 4.839, p <0.05]. Post hoc comparisons revealed that the concussed group with symptoms showed significant decreased changes of oxygenation during the memory task in the visual abstract design condition when compared with both of the other two groups (
Figure 7).
Post hoc comparisons for the concussed group with symptoms further revealed significant decreased changes of oxygenation during the memory task with the visual abstract designs when compared with the verbal stimuli.
Discussion
In the present study, we compared functional brain activation during the performance of a working memory task in a group of concussed subjects with severe postconcussive symptoms, with minor postconcussive symptoms and healthy controls using fNIRS. Our research revealed that during a working memory task, participants with a mTBI and persistent postconcussive symptoms 1) demonstrated decreased working memory functions; 2) showed decreased task-related brain oxygenation in frontal brain regions of both hemispheres compared with a healthy control group; and 3) showed decreased task-related brain oxygenation compared with concussed participants with minor postconcussive symptoms in the dorsolateral prefrontal cortex (BA 9/46) of the left hemisphere. The data further showed that 1) there was a significant negative correlation between the severity of postconcussive symptoms and brain oxygenation within the dorsolateral prefrontal cortex (BA 9/46) of the left hemisphere; 2) the dorsolateral prefrontal cortex (BA 9/46) of the right hemisphere had decreased oxygenation patterns for the concussed group with symptoms compared with each of the other two groups; and 3) the dorsolateral prefrontal cortex (BA 9/46) of the right hemisphere showed decreased oxygenation for the concussed group with symptoms during the memory task with visual abstract design compared with verbal stimuli.
The analysis of the performance scores for the working memory task revealed that the concussed group with severe symptoms answered incorrectly significantly more often than the control group. This is in contrast to previous findings from Chen et al.,
17 who did not find behavioral differences when using the same memory task. However, when looking at the individual level of performance scores of the concussed group in the study of Chen et al.,
17 there was great variability in the memory answers, ranging from chance to performance comparable to the control group. This might have accounted for the absence of significant differences between groups. In contrast, young concussed athletes performing the very same memory task demonstrated significantly poorer accuracy.
59 In fact, reduced working memory functions when suffering from postconcussive symptoms have been reported to be a common impairment after head trauma.
22,23,59,103–109 The present study groups underwent a detailed clinical examination by a psychiatrist who was also trained in neurology, psychosomatic medicine, and psychotherapy and were matched according to their age and BDI scores to account for influencing factors other than experienced concussions.
23,36,70,74,110 Thus, reduced memory functions are a significant factor when experiencing postconcussive symptoms, even long-term after the incident. In fact, the long-term effects regarding reduced memory functions in athletes with a history of mTBI have been reported previously in a study of subjects for whom more than 3 decades had passed since their last reported concussion.
39Analysis of the fNIRS data for working memory tasks revealed that compared with the control group, the concussed group with symptoms had significantly decreased changes of oxygenation within the DLPFC, frontopolar area, orbitofrontal area, and pars triangularis of the right hemisphere and the pars triangularis, pars opercularis, and DLPFC of the left hemisphere. Previous studies have shown altered brain activation following mTBI when using different memory tasks
8,18,55,56,70 but also when using the same working memory task.
17,59 However, whether brain oxygenation is increased or decreased after mTBI is a controversial topic. McAllister et al.
56 reported that mTBI patients showed increased activation patterns in response to moderate working memory processing loads, which were lateralized to the right hemisphere, including the DLPFC. In contrast, the same area showed decreased activation patterns in our subjects, which is in line with results from fMRI and PET studies.
17,18 In fact, Chen et al.
17 observed weaker BOLD changes within the DLPFC of the right hemisphere using the same memory task. Thus far, one study has used fNIRS to compare brain activation patterns of concussed patients and healthy controls.
70 The results showed significantly decreased oxygenation changes for a concussed group during a design working memory task over frontal regions of the right hemisphere. Thus, our results are consistent with previous findings indicating that persistent postconcussive symptoms are accompanied by decreased brain oxygenation in frontal brain regions during working memory tasks. However, the results of the present study suggest that decreased brain oxygenation due to persistent postconcussive symptoms is not limited to the DLPFC in the right hemisphere, but affects frontal regions, including the DLPFC of both hemispheres. We therefore suggest that fNIRS is not only a valid method for the investigation of mTBI, but more importantly, that the DLPFC of either hemisphere marks an area of functional differences between concussed subjects with persistent symptoms and healthy controls.
In contrast to previous findings revealed by fNIRS,
70 we found additional differences between the groups. Furthermore, group differences of the present findings were associated with the visual version as well as the verbal version of the memory task, a result that was not observed by Kontos et al.
70 Additional significant differences during verbal and visual working memory tasks were also found in the frontal brain regions of the left hemisphere (i.e., the pars triangularis [BA 45], pars opercularis [BA 44], and dorsolateral prefrontal cortex [BA 9/46]). Thus far, previous studies have reported altered brain activation during memory tasks primarily in the frontal cortices of the right hemisphere,
8,17,70 although it has been shown that the mid-DLPFC (BA 46/9) of both hemispheres is critically involved in memory functions.
91 Indeed, Keightley et al.
59 showed reduced brain activity in the DLPFC of both hemispheres in young concussed subjects (average age = 14.47 ± 2.3 years) using fMRI and the same memory task. The DLPFC demonstrated particular involvement in working memory tasks,
111–115 with a significant correlation between its activation and accuracy in memory tasks.
111 The present study, therefore, shows that concussed subjects that suffer from persistent postconcussive symptoms and decreased performance scores in working memory tasks also have decreased changes in oxygenation in the frontal cortices of either hemisphere when compared with healthy controls.
Comparisons further revealed decreased changes of oxygenation for the concussed group with severe postconcussive symptoms when compared with the concussed group with minor symptoms within one channel over the DLPFC of the left hemisphere. This indicates that a reduced hemodynamic response during memory tasks in concussed subjects with symptoms is tied to the presence or absence of postconcussive symptoms. Indeed, it has been shown that self-reported postconcussive symptoms are associated with an ongoing cerebral hemodynamic abnormality.
54,62 A correlation between self-reported symptoms and brain metabolism has also been documented using
1H-MR spectroscopy.
116 The present results show that concussed participants differ from concussed subjects with minor symptomatology by decreased brain oxygenation. Furthermore, the concussed group with fewer symptoms did not differ when compared with the healthy control group without a history of mTBI. It therefore seems that brain oxygenation within the left hemispheric DLPFC is closely related to the presence or absence of postconcussive symptoms. We therefore calculated a correlation analysis within the same channel, which revealed that changes of oxygenation and the severity of postconcussive symptoms are negatively correlated (i.e., the higher the severity of postconcussive symptoms, the less oxygenation changes within the dorsolateral prefrontal cortex [BA 9/46] of the left hemisphere during the performance of working memory tasks). This suggests that the presence and severity of postconcussive symptoms are important indicators and are related to altered brain oxygenation. Although self-reports of subjective symptoms are a reliable and accurate tool, brain imaging data are also of importance, as it is well known that a substantial number of athletes fail to report their symptoms to team officials.
117 For this reason, the present results suggest that fNIRS could be a potential tool in the diagnostics of mTBI, as brain oxygenation patterns correlate negatively with the severity of symptoms.
The DLPFC has been shown to be a critical brain region in the functional analysis of concussive symptoms in several studies.
17,55,56,70,59,118–120 However, previous studies provided inconclusive results regarding the question of whether the DLPFC is more affected within the right hemisphere
17,55,56,118,119 or bilaterally.
70,59 The present results suggest that decreased oxygenation of the DLPFC of the left hemisphere during memory tasks negatively correlates with symptom severity. Barbey et al.
113 showed in patients with lesions to the left hemispheric DLPFC that this brain region is necessary for manipulating verbal and spatial information in working memory. The present results indicate that the left hemispheric DLPFC is sensitive to functional alterations as a consequence of mTBI and therefore might be a potential region to gain objective data in order to distinguish between concussed subjects with severe or mild symptomatology.
Furthermore, the concussed group showed decreased brain oxygenation within the DLPFC of the right hemisphere (BA9/46) during the memory task with the visual abstract designs when compared with either group. The concussed group with symptoms showed significantly less brain oxygenation within the DLPFC of the right hemisphere during the memory task with the visual abstract designs compared with the verbal design. Thus far, there are no previous reports of specific decreases in the right hemisphere when memory tasks rely on visual abstract designs; however, it has been documented that the visuospatial abilities of the right hemisphere exceed those of the left hemisphere.
121–123 Corballis
124 described the right hemisphere as more visually intelligent than the left one and suggested the existence of a right hemisphere “interpreter” of higher-level visual mechanisms. Therefore, the results of the present study suggest that hemispherically specialized functions of visual abstract memory abilities should be addressed in future studies of mTBI.
Conclusions
Based on the pattern of results found in the present study, we conclude that impaired memory functions and decreased brain oxygenation within the DLPFC of either hemisphere using fNIRS characterize concussed subjects who suffer from long-term postconcussive symptoms compared with healthy controls. The DLPFC of the left hemisphere seems to be related to severity of symptoms and can therefore serve as a potential brain region to distinguish between concussed subjects with severe or mild symptomatology. The DLPFC of the right hemisphere instead has shown to correlate more with decreased brain oxygenation in concussed subjects during memory tasks with visual abstract designs. Future studies should therefore take into account that mTBI, in particular, might alter higher-order brain functions that are hemispherically specialized. Hence, persistent post-concussive symptoms are related to decreased brain oxygenation during a working memory task within the DLPFC. fNIRS can be considered as a useful instrument in future research of mild traumatic brain injury.
Acknowledgments
Previous Presentation: fNIRS 2014, October 10–12, 2014, Montreal, Quebec, Canada.
The study was funded by the University research funding of the German Sports University, Cologne and by the Heinrich Hertz Foundation, Ministry of Innovation, Science and Research of the German State of North Rhine-Westphalia (B 41 Nr. 6/13).