The Psychiatric Phenotype of Anti-NMDA Receptor Encephalitis
Abstract
Methods
Patients
Exclusion Criteria
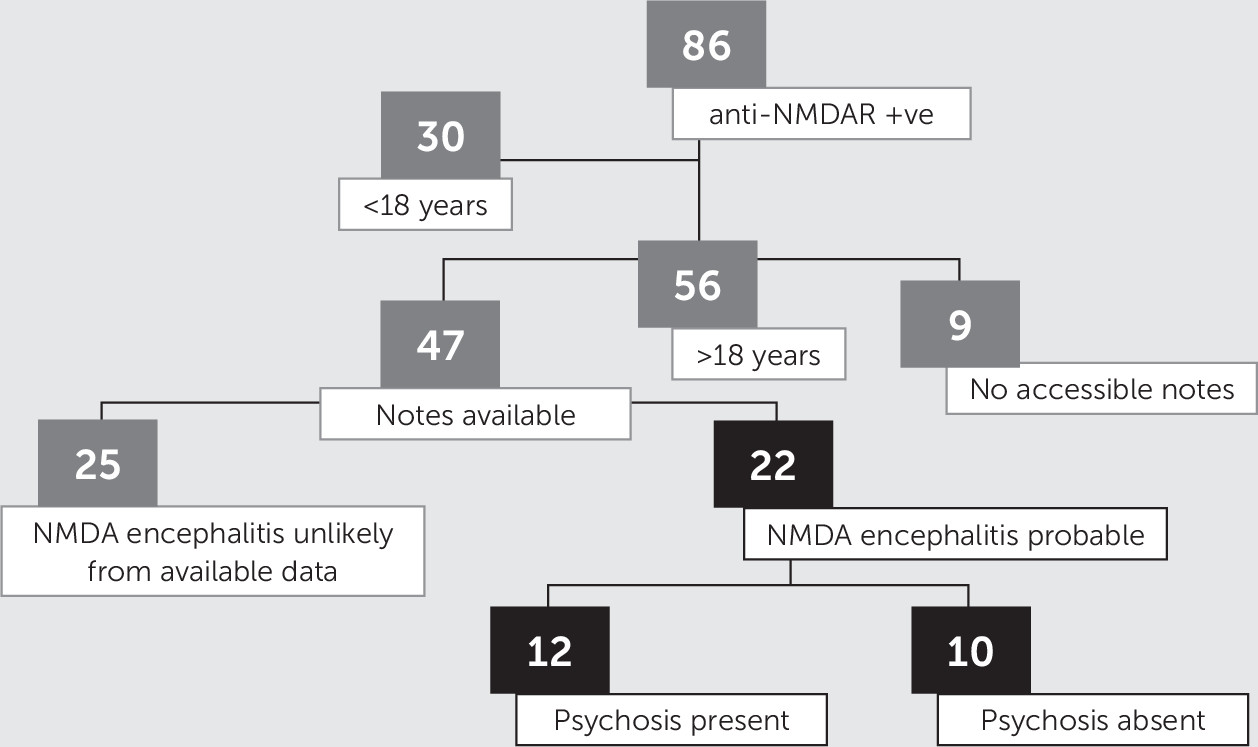
Inclusion Criteria
| Patient Number, Gender, Age (years) | Psychosis | Speech Disturbance | Seizure | Movement Disorder | Reduced Consciousness | Autonomic Dysfunction | Teratoma | EEG (Abbreviated Reports) | Tested Serum Antibody | CSF | MRI (Abbreviated Reports) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4, Female, 76 | Nonpsychotic | + | – | + | + | – | – | Dominant theta background; bursts of bitemporal slow waves, left predominant | NMDAR + VGKC – | Normal | Mild cerebral small vessel disease |
| 1, Male, 77 | Nonpsychotic | + | + | + | + | + | – | Periodic temporal sharp waves and polyspikes. Diffuse low amplitude delta activity with intermixed fast activity | NMDAR+ VGKC 101 | Normal | Diffuse T2 hyperintensity and swelling; left hippocampus, cortical/subcortical inferior frontal and temporal, insula, pulvinar; mature left middle cerebral artery territory infarct |
| 2, Male, 40 | Nonpsychotic | + | – | + | + | + | – | Slow background activity with frequent bifrontal sharp delta waves. Triphasic at times | NMDAR + VGKC – VGCaC – Ro/SSA + TPO – | WCC 25, 94% lymph | Diffuse T2 hyperintensity of amygdala, hippocampus, splenium of corpus callosum and periventricular white matter bilaterally |
| 13, Female, 27 | Nonpsychotic | + | + | + | – | – | – | Runs of theta slowing and sharp waves left temporal | NMDAR + ANA – | Normal | Normal |
| 16, Female, 71 | Nonpsychotic | + | + | + | + | + | – | Nearly continuous periodic sharpened slow waves (PLEDs) left temporal, parietal and occipital | NMDAR + LGI1 –CASPR2 – TPO 61 ANA – ds DNA – | OCB matched | T2 hyperintensity cortical/subcortical in posterior temporal and parietal lobes, left temporal lobe most extensive |
| 9, Female, 54 | Nonpsychotic | – | + | + | + | – | – | Mild, diffuse background slowing | NMDAR+ VGKC 277 Hu – Yo – | N/A | Nonspecific scattered foci of T2 high signal in cerebral white matter bilaterally |
| 23, Male, 42 | Nonpsychotic | + | + | + | – | – | – | Intermittent bitemporal slowing with spikes and sharpened slow waves | NMDAR+ GAD + | OCB unmatched | Resection cavity in right temporal lobe; Known temporal lobe glioma resection ≥20years prior |
| 24, Female, 69 | Nonpsychotic | + | – | – | + | + | – | Frequent slow/sharp waves, bifronto-temporal, left emphasis | NMDAR + Hu – Yo – | N/A | Generalized volume loss. |
| 15, Male, 72 | Nonpsychotic | + | – | + | + | – | – | Diffusely slow background, left temporal focal slowing | NMDAR + VGKC – | Normal | Extensive T2 hyperintensity in insula, parietal and lateral temporal lobe |
| 49, Female, 44 | Nonpsychotic | + | – | + | + | + | – | Bifrontal and left temporal sharp and slow waves | NMDAR+VGKC 101 Hu – Yo – | Normal | T2/flair hyperintensity bilaterally in caudate heads, putamina, globus pallidus, midbrain and right tail of hippocampus |
| 6, Female, 29 | Psychotic | + | + | + | + | + | + | Slowed background activity, frequent left temporal sharp waves | NMDAR + VGKC – ANA – TPO – dsDNA – | WCC 31 94% lymph | Normal |
| 7, Female, 36 | Psychotic | + | – | + | + | + | + | Normal | NMDAR + VGKC –VGCaC – ANCA – dsDNA – GAD low + Hu – x2028;Yo – | OCB unmatched | Small area of cortical T2 high signal in the posterior right cerebellar hemisphere |
| 12, Female, 22 | Psychotic | + | + | + | + | + | + | Diffuse bilateral left predominant fronto-temporal slow wave with sharpened theta activity | NMDAR + ANCA –dsDNA – GAD – | WCC 30 | Diffuse cerebral edema and mild bilateral uncal herniation; diffuse leptomeningeal enhancement |
| 14, Female, 45 | Psychotic | + | + | + | + | – | – | Theta and delta transients mainly left temporal | NMDAR + | IgG 37 | Normal |
| 17, Female, 22 | Psychotic | + | + | + | + | + | + | Right fronto-temporal sharp and slow waves and independent left sharp transients | NMDAR + VGKC – ANCA – dsDNA – GAD – ganglioside – | Protein 0.47g/dL | Normal |
| 18, Female, 23 | Psychotic | + | – | + | + | + | – | Diffusely slow background with frontal bilateral high amplitude delta slow waves | NMDAR + (CSF and serum) VGKC – | Normal (no OCB, WCC<5) NMDA + | Normal |
| 19, Female, 32 | Psychotic | + | + | + | – | – | – | Abnormal (report unavailable) | NMDAR + (CSF and serum) VGKC – | OCB unmatched NMDA+ | Left mesial temporal sclerosis |
| 5, Female, 52 | Psychotic | + | – | + | – | + | –Ovarian cyst | Sharp spikes in temporal area | NMDAR + VGKC – Hu – Yo – | Normal | Mild small vessel disease cerebral hemispheres |
| 11, Female, 19 | Psychotic | + | – | + | – | + | + | Normal | NMDAR + VGKC – Hu – Yo – | Normal | Normal |
| 20, Male, 56 | Psychotic | + | + | – | – | + | – | Focal slowing over left temporal with sharp waves seen intermixed and independently | NMDAR+VGKC 193 Hu – Yo – | Normal | Normal |
| 27, Female, 33 | Psychotic | + | – | + | – | + | – | Not available | NMDAR +VGKC – TPO – | N/A | N/A |
| 48, Male, 37 | Psychotic | + | + | + | – | – | – | Frequent right temporal delta brush activity | NMDAR + | OCB unmatched | T2 hyperintensity and volume loss, right temporal pole and hippocampus; recent HSV encephalitis |
Data Collection
Measures
Statistical Analyses
Results
Descriptive Data
Clinical symptoms.
Investigations.
Psychosis
| Characteristic | With Psychosis (N=12) | Without Psychosis (N=10) | p | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Female | 10/12 | 83 | 6/10 | 60 | 0.348 |
| Psychiatric admission | 7/12 | 58 | 0/10 | 0 | 0.005 |
| Psychiatric review | 12/12 | 100 | 4/10 | 40 | 0.003 |
| Presence of teratoma | 5/12 | 42 | 0/10 | 0 | 0.040 |
| Seizures present | 7/12 | 58.3 | 5/10 | 50 | 1.00 |
| Movement disorder | 11/12 | 91.7 | 9/10 | 90 | 1.00 |
| Autonomic instability | 9/12 | 75 | 5/10 | 50 | 0.378 |
| Reduced consciousness | 6/12 | 50 | 8/10 | 80 | 0.204 |
| Abnormal EEG | 9/11 | 82 | 10/10 | 100 | 0.476 |
| CSF: pleocytosis or oligoclonal bands | 5/11 | 45 | 3/8 | 38 | 1.00 |
| MRI suggestive of encephalitis | 3/11 | 27 | 4/10 | 40 | 0.659 |
| Mean | SD | Mean | SD | ||
| Age (years) | 33.8 | 12.0 | 57.2 | 18.0 | 0.003 |
| MRS at nadir | 4.0 | 0.95 | 3.9 | 1.4 | 0.842 |
Psychosis subgroup: PANSS symptom profile.
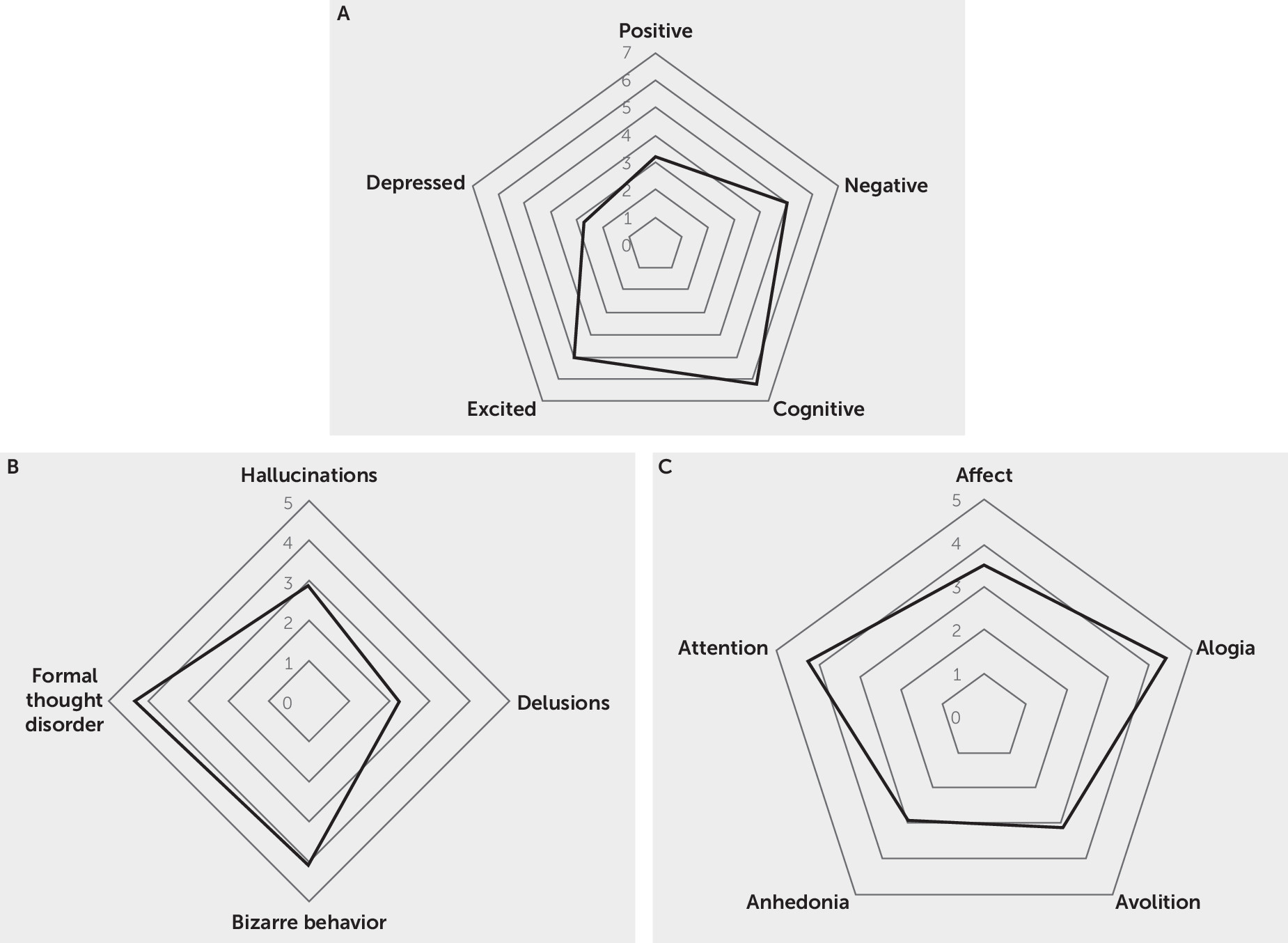
Psychosis subgroup: SAPS and SANS profile.
Nonpsychosis subgroup: symptom profile.
Discussion
Limitations
Conclusions
References
Information & Authors
Information
Published In
History
Keywords
Authors
Competing Interests
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

