Patients with refractory temporal lobe epilepsy (TLE) who undergo temporal lobectomy may experience a spectrum of psychopathological disturbances postoperatively (
1,
2). It is important to understand the neuropathological underpinnings and the impact of this psychopathology so that clinicians are better able to prevent and manage this disorder (
1,
2). Postoperative mania is relatively infrequent, seen in only 5%−10% of cases (
2,
3). Previous studies have shown risk factors and suggested potential mechanisms for the emergence of mania postoperatively (
3); however, the neuropathogenesis remains incompletely understood. Risk factors include residual seizures postoperatively, generalized tonic-clonic seizures, presence of bilateral EEG abnormalities, and right (rather than left) temporal lobectomy (
3). White matter dysfunction has been postulated as a potentially important contributor to the pathogenesis of mania in this setting. Here, we report a case of mania after left temporal lobectomy that was associated with bilateral white matter dysfunction, with involvement of the ipsilateral uncinate fasciculus. Additionally, we discuss evidence of the role that white matter dysfunction (and uncinate fasciculus injury in particular) played in generating the patient’s postoperative mania by altering mood and reward regulation processes and provide suggestions regarding how the risk of postoperative mania may be predicted in this setting.
Case Report
A 58-year-old, right-handed man was diagnosed with epilepsy at age 12. His seizures were stereotypically characterized at onset by a rising epigastric sensation, loss of awareness, lip smacking, and repetitive bilateral hand movements. Each episode lasted 2–3 minutes, followed by 15–20 minutes of confusion. The maximum seizure frequency was multiple times daily, and the minimum was once per month. The seizures occasionally evolved into generalized tonic-clonic seizures. Between episodes, the patient experienced attention, memory-retrieval, and word-finding difficulties. His past medical history included infrequent episodic migraines without aura. He had no obvious seizure risk factors during childhood and no history of traumatic brain injury. He did not smoke, drink, or use illicit substances. His past psychiatric history was significant for a transient episode of behavioral dysregulation after a cluster seizure, characterized by irritability, mood lability, insomnia, logorrhea, hypermotoricity, and excessive libido.
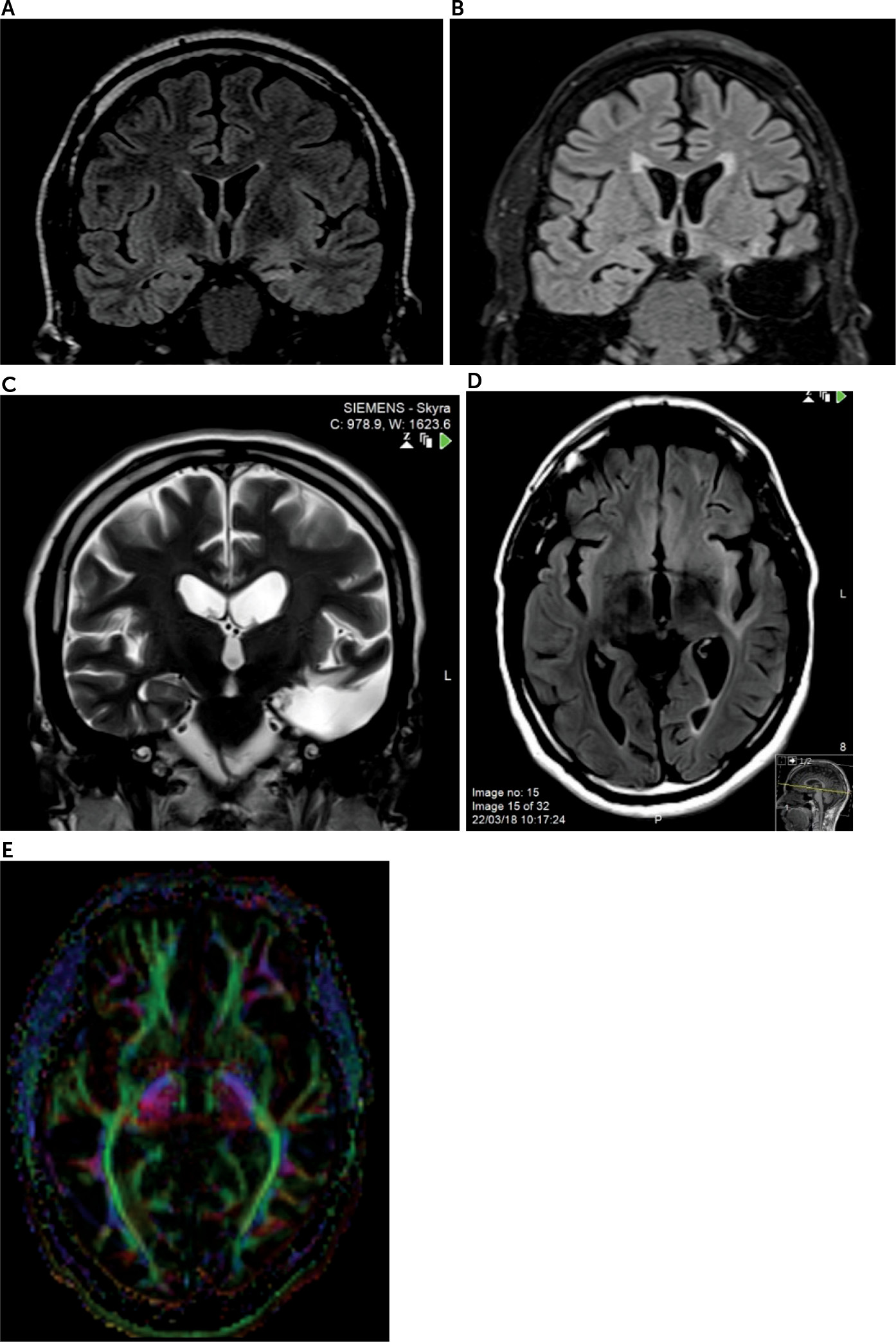
After adherence to multiple antiepileptic drugs in various combinations for years, his episodes were deemed medication-refractory. He was monitored with video EEG, which captured three electrographic seizures with left anterior temporal onsets and a consistent seizure semiology. There were no right-sided or extratemporal EEG abnormalities. Brain MRI showed left mesial temporal sclerosis and mild temporal atrophy (
Figure 1A). The patient underwent an uncomplicated left anterior temporal lobectomy. Histopathological examination revealed hippocampal sclerosis, focal cortical dysplasia type 1A, subpial gliosis, and oligodendrocyte hyperplasia.
The patient’s seizures stopped after his surgery, and he remained seizure-free (for the 2 years since the surgery). He adjusted well in the immediate postsurgical period, except for moderate worsening of presurgical word-finding and memory retrieval difficulties. Three months after the surgery, he became persistently euphoric and disinhibited and exhibited socially inappropriate behavior. He spoke excessively, interrupting others, and used profane language in front of children. A native Arabic speaker, he started speaking English in social gatherings to emphasize his exceptional education. He frequently remarked that he was not afraid of law enforcement. He had excessive energy, requiring only 2–3 hours of sleep per night, and consumed an average of eight meals per day. He impulsively spent a significant amount of his family’s savings on furniture, clothing, and travel. He became hyperreligious and often spoke of a special connection with God. He became uncharacteristically hypersexual and even stalked a local female dentist. Although he was previously devoted to his wife, expressing gratitude for her support during his illness, he became emotionally detached from her and vehemently denied that she had helped him during the course of his epilepsy. In contrast, he commended his health care team. A neuropsychiatric evaluation confirmed that the patient met criteria for a nonpsychotic manic state. He had no insight that these behavioral changes were uncharacteristic, nor did he appreciate how his behavior had affected him and those around him.
Video EEG monitoring and several routine EEGs during his period of mania showed occasional left temporal theta slowing without epileptiform abnormalities. Postoperative MRI showed excision of the left anterior temporal lobe without significant residual mesial temporal tissue and with adjacent postsurgical T
2 and fluid-attenuated inversion recovery hyperintense signal (
Figure 1B). In addition, abnormal signal hyperintensities were observed in subcortical white matter, particularly involving temporal, peri-insular, and orbitofrontal regions (
Figure 1C and 1D). Tracts that likely passed through the regions showing abnormal signal include the left uncinate fasciculus, possibly along with the inferior occipital fasciculus, arcuate fasciculus, and other tracts. These findings were new since the preoperative MRI. Postoperative diffusion tensor imaging (DTI) showed low overall fractional anisotropy values bilaterally, with a reduction in left-sided fractional anisotropy values, compared with the right side (
Table 1) (
Figure 1E).
During this time, the patient was taking topiramate (200 mg b.i.d.) and oxcarbazepine (600 mg t.i.d.). Treatment with quetiapine was commenced and titrated upward to a daily dose of 600 mg. Topiramate was gradually withdrawn and discontinued over the course of the next year. Oxcarbazepine was maintained due to its mood-stabilizing properties. The patient’s symptoms significantly improved and then abated with treatment. After 6 more months, quetiapine was tapered down and discontinued. The patient has been stable without recurrence of mania for a year after discontinuing these medications.
Discussion
Several mechanisms may lead to mania following temporal lobectomy (
2). Residual seizure activity leading to ictal or postictal mania is one potential mechanism (
4). Our patient’s EEG during mania was devoid of evidence supporting the residual seizure hypothesis. His mania may have been related to EEG-negative, simple partial seizures; however, the absence of other manifestations pathognomonic for seizure despite the prolonged duration of the mania makes this unlikely.
White matter dysfunction has been postulated as a potentially important mechanism that may predispose patients with TLE to mania (
3). The patient’s bilateral fractional anisotropy values were low compared to patients with TLE who underwent temporal lobectomy (
5). Widespread white matter dysfunction is well known to occur in patients with TLE pre- and postoperatively (
5). This may be partly attributable to the epilepsy itself, in addition to the progressive degeneration of surgically severed or distorted white matter tracts (
5). White matter dysfunction may disrupt cortical-subcortical circuits responsible for mood regulation and reward processing (
6,
7). Mood regulation depends on the integrity of prefrontal-amygdala-hippocampal circuits (
7). The orbitofrontal cortex (OFC), mediodorsal prefrontal cortex (PFC), and anterior cingulate cortex play a key role in automatic (implicit) mood regulation, whereas the dorsolateral PFC and ventromedial PFC (vmPFC) play a role in voluntary mood regulation (
7). Reward processing, on the other hand, depends on the integrity of PFC-striatal circuits (
7). White matter injury may impair communication between prefrontal hubs of these circuits and their subcortical components. This may underly the episodes of mania in our patient. It has also been proposed as a neuropathogenic mechanism in bipolar affective disorder.
In the above case, it is noteworthy that the left uncinate fasciculus appeared particularly damaged when its MRI signal characteristics and fractional anisotropy values were compared with its right counterpart. The uncinate fasciculus is an associative, limbic white-matter tract that bidirectionally connects the OFC with anterior mesial temporal structures (
8). It is thought to play a role in memory and word retrieval (
8). It has also been proposed that the uncinate fasciculus integrates input from learning, emotion, and memory processes (subserved by the mesial temporal structures) with mood regulation and reward assignment (subserved by the OFC and vmPFC) (
8). Injury to the uncinate fasciculus in the above patient, along with the excision of anterior temporal structures, may have deprived the mood-elevating left OFC of input from remaining left temporal regions. A previously described model for bipolar affective disorder similarly implicated overactivity in the left OFC and vmPFC and subtle structural damage to the mesial temporal lobe (
7). Patients with bipolar affective disorder were found to have lower fractional anisotropy values in the uncinate fasciculus compared with healthy control subjects and patients with unipolar depression (
9). The uncinate fasciculus has also been shown to be compromised in patients with affective psychosis (
10). Although a certain degree of injury to the uncinate fasciculus is unavoidable in most cases of anterior temporal lobectomy due to the excision of the mesial temporal origins of the tract (
11), and the majority of these cases are not complicated by full-blown postoperative mania, the neuropsychiatric sequelae of varying degrees of uncinate fasciculus injury have not been sufficiently studied (
8). As such, the spectrum of potential sequelae of uncinate fasciculus injury, including mood dysregulation, may be underrecognized and underreported (
2,
8). The severity of our patient’s symptoms may relate to the degree of injury to the uncinate fasciculus, although this remains speculative. Our patient also manifested additional potential evidence of uncinate fasciculus dysfunction, including word-finding and memory retrieval difficulties. Interestingly, his denial to show gratitude toward his wife and his emotional detachment from her may be understood as a form of Capgras syndrome. The uncinate fasciculus, hypothesized to assign emotion to mnemonic and semantic memory, has been linked to this syndrome as well (
8).
It is important for clinicians to anticipate postsurgical neuropsychiatric complications in patients with TLE. It appears that the most significant risk factor is the presence of preoperative neuropsychiatric symptoms (
12). These may indicate preexisting, potentially widespread cerebral dysfunction (
12). Bilateral EEG abnormalities, another indicator of widespread cerebral dysfunction, is a risk factor for postictal mania (
4). The patient in the above case exhibited presurgical psychiatric problems. His EEG did not show bilateral cerebral dysfunction, but DTI results did (albeit postoperatively). This suggests that bilateral DTI abnormalities may be a predictive biomarker for the risk of postsurgical mania.
In conclusion, this case demonstrates the potential underlying neuropathological mechanisms and risk factors of mania in this setting, including the potential role played by white matter dysfunction in altering mood regulation and reward circuitry. Further studies are needed to accurately reveal the neuropsychiatric sequelae of dysfunction of specific white matter tracts, such as the uncinate fasciculus, and to investigate the potential yield of evaluating white matter integrity with DTI to predict the occurrence of postictal mania. It is important to examine how neuropsychiatric risk factors and outcomes may be integrated in the decision-making process and preoperative work-up for patients with epilepsy so that the risks and benefits of epilepsy surgery are objectively evaluated (
13).