Repetitive transcranial magnetic stimulation (rTMS) is a neuromodulatory technique with antidepressant (
1) and neurorehabilitative effects (
2). rTMS selectively modulates cortical excitability (
3), which is often affected in traumatic brain injury (TBI) (
4). rTMS has thus been proposed as a potential treatment for patients with TBI-associated depression (
5), especially given that these patients may be less responsive to antidepressant pharmacotherapy (
6). While concern for rTMS-induced seizure risk often limits its use in TBI, this risk appears to be elevated primarily in penetrating-hemorrhagic injuries rather than diffuse-multifocal axonal injury (
7).
rTMS for major depressive disorder is traditionally targeted to scalp regions approximately overlying dorsolateral prefrontal cortex (DLPFC) (
1), which likely modulates activity in the subgenual anterior cingulate cortex (sgACC) (
8). This concept dates back to the successful use of rostral cingulotomy, a neurosurgical procedure that was first used in the 1950s to disrupt connectivity between prefrontal cortex and deep limbic regions (
9). Subsequent neuroimaging studies confirmed that depression is associated with hyperactivity in sgACC and anticorrelated hypoactivity in DLPFC (
10). This sgACC hyperactivity appears to be normalized when antidepressant pharmacotherapy (
11) and electroconvulsive therapy (
12) are effective but not when these treatments are ineffective. In light of these findings, deep brain stimulation (DBS) has successfully been used to treat depression by directly attenuating sgACC hyperactivity (
13).
Similar principles have also been proposed for identifying rTMS targets. Across a wide variety of neuropsychiatric disorders, functional connectivity studies show that optimal targets for excitatory rTMS appear to be functionally anticorrelated with optimal DBS targets for the same disorder (
14). In the case of major depression, rTMS response is associated with connectivity between the DLPFC stimulation site, sgACC, and the default mode network (DMN) (
8,
15–
17).
Although functional connectivity-based targeting of regions anticorrelated with sgACC has demonstrated promise in large-group studies, this approach has thus far been unsuccessful for identifying patient-specific treatment targets (
14,
16,
18). This may be explained by interindividual variability in functional localization of resting state networks (RSNs), a series of large-scale brain systems composed of various anatomical regions that coordinate to perform specific functions (
19,
20). Such variability in RSN anatomy is not reliably identified by functional connectivity with seeds derived from group averages (
21). The recent advent of iterative correlation and classification algorithms, which can reliably partition a human brain into several individualized RSN maps (
21–
23), may therefore enable development of patient- and disease-specific rTMS targeting.
Because recent cortical topographic maps classify sgACC as part of DMN (
24), mapping this network may serve as a reasonable method for determining individualized sgACC connectivity profiles. DMN is most strongly anticorrelated with dorsal attention network (DAN), which contains DLPFC nodes that show substantial interindividual topographic variability (
20). In major depression, these DLPFC regions show hypoconnectivity with DAN and hyperconnectivity with DMN (
25). DBS of sgACC has been shown to modulate interactions between attention-switching and self-referential emotional engagement, functions that are mediated by DAN and DMN, respectively (
26). Due to these parallels with the aforementioned DLPFC-sgACC anticorrelation in major depression, mapping DAN-DMN anticorrelation may serve as a reasonable proxy for identifying individualized rTMS targets that will modulate DLPFC-sgACC interactions.
Individualized functional localization is particularly challenging in TBI, which causes multifocal white matter injury (
27) and less predictable functional connectivity changes (
28). However, there is a need for neuromodulatory treatment approaches given the lack of effective antidepressant treatments for TBI-associated depression (
6). Like major depression, TBI can affect functional connectivity in anterior cingulate cortex (ACC), DLPFC, DAN, and DMN (
29–
33). Repetitive TBI has additionally been associated with neurodegenerative changes in medial temporal lobe (MTL) on histopathology and positron emission tomography (
34,
35). Due to these changes, patients with repetitive head trauma provide a unique opportunity to investigate not only the nature of the circuitry underlying its neuropsychiatric phenotype, but also into the nature of brain network dynamics in response to noninvasive brain stimulation. Thus, we hypothesized that stimulating the DLPFC target with maximal subject-specific DAN-DMN anticorrelation would modulate sgACC and improve mood in repetitive TBI. Here, we report a single case in which this approach was successfully implemented in a patient with repetitive TBI.
Methods
Standard Protocol Approvals, Registrations, and Participant Consents
This report reflects results observed in a single subject who was part of a pilot randomized-controlled trial with a planned sample size of 20. The trial was conducted in accordance with a protocol approved by the Human Research Protection Office at Washington University School of Medicine in St. Louis. All individuals gave informed written consent. The study was reviewed regularly by the investigators for safety.
Participants
Patients.
We evaluated a man in his fourth decade of life with a history of neuropsychiatric illness associated with repetitive head trauma during a prior career as a defensive lineman in the National Football League (NFL). He recalled a history of at least 12 prior concussions, including at least two at the amateur level and at least 10 in the NFL. He likely experienced at least 7,000 subconcussive head impacts before his NFL career, as estimated by the Cumulative Head Impact Index (
36), and an unknown but likely comparable number during his NFL career.
He described a 2- to 3-year history of progressively worsening depression, anxiety, impulsivity, anger, and cognitive impairment (particularly long-term and short-term memory). He was unable to work and had restricted social function. He had previously demonstrated inadequate response to sertraline, paroxetine, and alprazolam and was not taking any neuropsychiatric medications at the time of the study. His structural MRI scan was within normal limits (for further details, see Figure S1 in the online supplement).
Patients with TBI-associated depression.
Ten additional subjects with a history of depression and TBI (eight males, ages 19–64) received rsfMRI scans as part of the aforementioned randomized-controlled trial with the same imaging protocol as the experimental subject. This included patients with a score of at least 10 on the Montgomery-Åsberg Depression Rating Scale (MADRS) and a history of concussive TBI, for which they were referred to the Washington University TBI clinic.
Healthy comparison subjects.
The healthy group included 12 male volunteers with no reported TBI history who received rsfMRI scans as part of the Human Connectome Project (HCP) 900-subject release (
37). Because HCP subjects are limited to 35 years of age at the time of enrollment, perfect age-matching was not possible. Instead, approximate age-matching was attempted by including all four male subjects in the ≥36-year-old age group as well as eight random subjects in the 31- to 35-year-old age group.
Clinical Assessments
Clinical testing at baseline and after the full course of treatment included depression testing with MADRS; personality testing with the Temperament and Character Inventory (TCI); self-report mood scales in the National Institutes of Health (NIH) Toolbox Emotion Battery (EB) and TBI Quality of Life Scale (TBI-QoL); cognitive testing with the NIH Toolbox Cognitive Battery (CB); self-report headache Likert scores and six-question Headache Impact Test (HIT-6); and an expert psychiatric evaluation on the basis of DSM-5 diagnostic criteria. Structural and functional MRI scans were performed at baseline and at the end of the treatment course. Unblinded MADRS, TCI, and EB were repeated at a follow-up assessment 6 weeks after the completion of the treatment course; CB and TBI-QoL were not repeated due to the subject’s preference. MADRS was the primary outcome measure for the double-blind randomized-controlled trial.
MRI Acquisition and Analysis
Full acquisition and preprocessing parameters are presented in the supplement. Pre- and posttreatment MRI included one T
1-weighted sequence and 16.5 minutes of blood oxygen level dependent (BOLD) fMRI sequences, which were collected using a 3-T Siemens Magnetom Prisma magnetic resonance scanner (Siemens, Erlangen, Germany). Additional scans were also collected immediately before and after the sixth and 15th treatments. Preprocessing and quality assurance were conducted following Power et al. (
38) (for further details, see Figure S2 in the
online supplement).
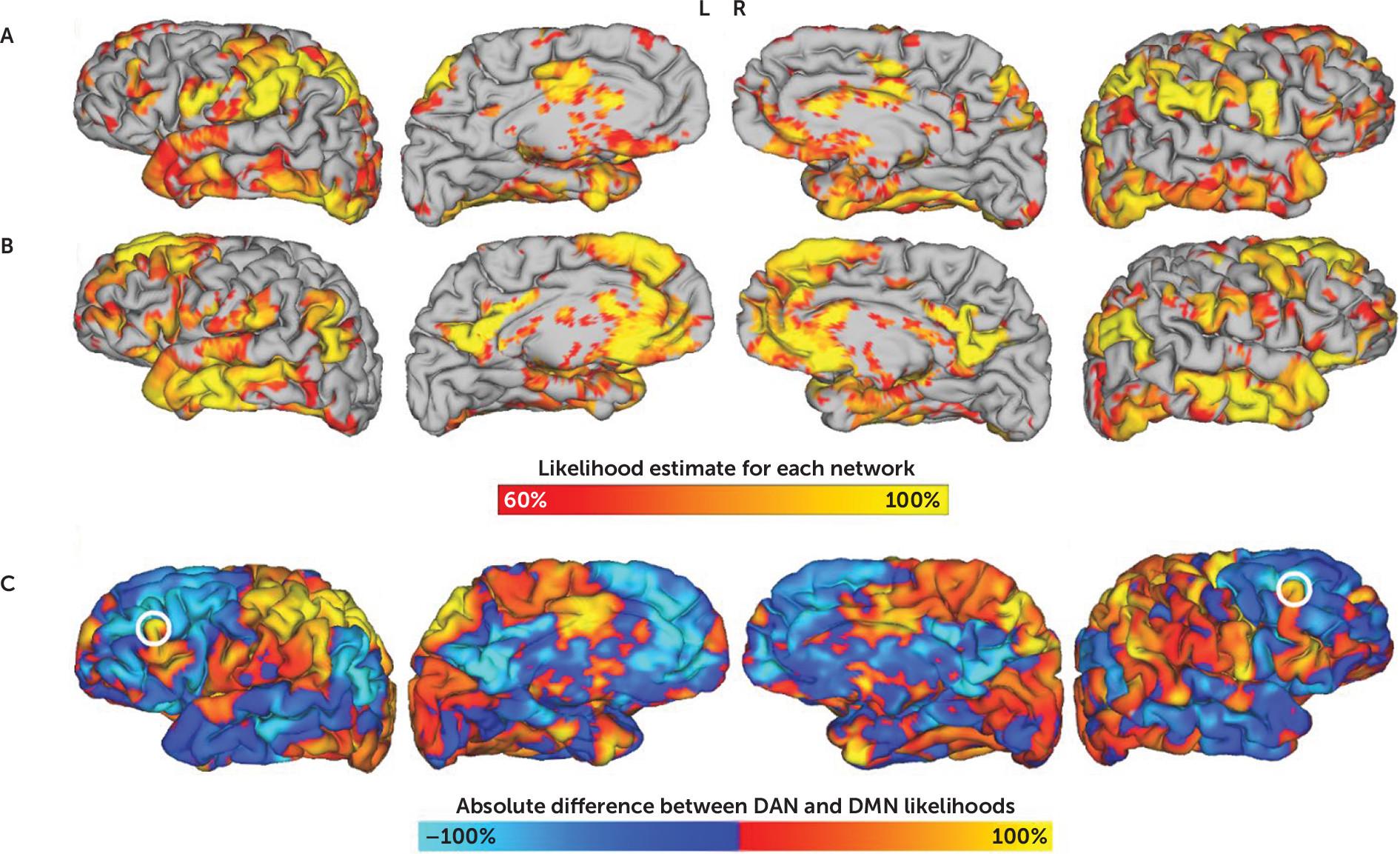
BOLD timecourses were used to construct individual-level resting-state network maps via the multilayer-perceptron-based machine learning classifier described in Hacker et al. (
21). This algorithm was initially trained on a reference set of correlation maps derived from seed locations identified by task meta-analyses. For new subjects, the trained classifier determines the likelihood of each voxel’s membership in one of seven cortical networks (dorsal attention, ventral attention/cingulo-opercular, frontoparietal control, default mode, motor, language, and visual) on the basis of its voxel-wise correlation map.
rTMS Target Selection
On the basis of the individualized network maps, a separate map was computed to reflect the absolute difference between DAN and DMN (
Figure 1). The image was masked to include only voxels within 6 mm of the dural surface, as deeper regions are less accessible via rTMS. As an approximation of DLPFC, a second mask was applied to include only voxels within 20 mm of previously reported coordinates (
16) for Brodmann areas 9 and 46. Although prior retrospective work has used a 25-mm radius (
16), this was less practical for a prospective study due to the risk of stimulating ventrolateral regions, which are more likely to cause painful contraction of masseter and temporalis muscles. Positive clusters in the resulting image were identified using FSL’s cluster algorithm with an image threshold of 75% of the maximum Z-score (FMRIB Software Library, Oxford, United Kingdom) (
39). The centers of gravity of the peak clusters in each hemisphere were selected as the optimal left- and right-sided rTMS stimulation coordinates (
Figure 1).
These coordinates were transformed to native space and identified on a surface reconstruction of the subject’s structural MRI scan using the Brainsight neuronavigation system (Rogue Research, Montreal). An optimal stimulation trajectory was chosen as a vector perpendicular to the dural surface at the target coordinate. Stimulation was guided along this trajectory using frameless stereotactic neuronavigation. The magnetic coil was adjusted in real time when the subject’s head motion caused greater than 5 mm of displacement from the target, which sometimes required slight modification of the angle between the trajectory vector and the dural surface.
Targeting Method Comparison
Alternative target coordinates were generated on the basis of three previously described methods:
1.
“5-cm rule”: This method, commonly used in clinical practice, identifies a target 5 cm anterior to the location at which single-pulse motor cortex stimulation induces a contraction of the contralateral abductor pollicis brevis muscle. Because this approach does not allow for calculation of precise target coordinates, we used the estimates determined in Fox et al.’s prior study of rTMS targeting (
16).
2.
Structural MRI-based targeting: This method uses DLPFC coordinates that have been used for targeting in recent large multicenter trials of neuronavigated rTMS (
40). Although other anatomical targets have been used in the past, recent consensus is converging toward the use of coordinates with maximal normative anticorrelation with sgACC (
15,
16).
3.
Individualized anti-sgACC target: This method relies on an individual subject’s anticorrelation with a group-mean definition of sgACC (
15,
16). A whole-brain correlation map for a seed in the sgACC (for further details, see Table S1 in the
online supplement) was masked to include only cortical regions within 20 mm of previously reported coordinates (
16) for Brodmann areas 9 and 46. The peak negative cluster in this map was identified using FSL’s cluster algorithm. The center of this cluster was considered to be the anti-sgACC rsfMRI-based target.
Spatial distance was compared for left- and right-sided targets between these three methods and our novel targeting method.
Functional Connectivity Analyses
A whole-brain “winner take all” parcellated map was generated by assigning each voxel to the network at which it demonstrated the highest likelihood of membership. These maps were used to subjectively compare RSN topography between the experimental subject, the group of healthy comparators, and a representative example of a healthy comparator (for further details, see Figure S4 in the online supplement). The seven networks identified by this parcellated map were used as regions of interest for seed-based connectivity analysis.
Treatment-induced changes were determined using conventional methods for seed-based functional connectivity analysis (supplementary methods, section 2.1). Predefined seed pairs included DAN to DMN, sgACC to DMN, medial orbitofrontal cortex (mOFC) to nucleus accumbens (NAcc), and MTL to DMN. Seed-based correlation was also assessed with several exploratory regions, including lateral orbitofrontal cortex, ventral attention network, and left-right rTMS stimulation sites (for further details, see Table S1 in the online supplement). These values were compared between pretreatment scans, posttreatment scans, and both comparator groups.
For the additional rsfMRI scans before and after the sixth and 15th treatments, treatment-induced changes in connectivity were compared in an exploratory manner in order to investigate potential mechanisms of treatment.
rTMS Treatment
The subject was randomly assigned to active treatment and received 20 sessions of bilateral rTMS using high-frequency left-sided stimulation (4,000 pulses at 10 Hz frequency with 5-second trains and 20-second intertrain interval) and low-frequency right-sided stimulation (a single train of 1,000 pulses with 1 Hz frequency) with a Magstim Rapid
2 stimulator and 70 mm air-cooled coil. The intensity of rTMS stimulation was 120% of resting motor threshold determined using the TMS Motor Threshold Assessment Tool (
41).
Results
Clinical Response
MADRS score improved by 72% with treatment and remained at the same level upon 6-week follow-up. Secondary outcome variables, including personality scales, cognitive test scores, and self-report emotion scales, are summarized in
Figure 2. The subject experienced no seizures, headaches, or other persistent adverse effects. Headache Likert score improved from 5/10 to 3/10, whereas self-report HIT6 score improved from 74 to 64. Transient twitching of facial muscles occurred during treatment, but this was not associated with pain or persistent discomfort. He also incidentally reported a reduction in nicotine cravings and successfully discontinued cigarette use over the course of the study.
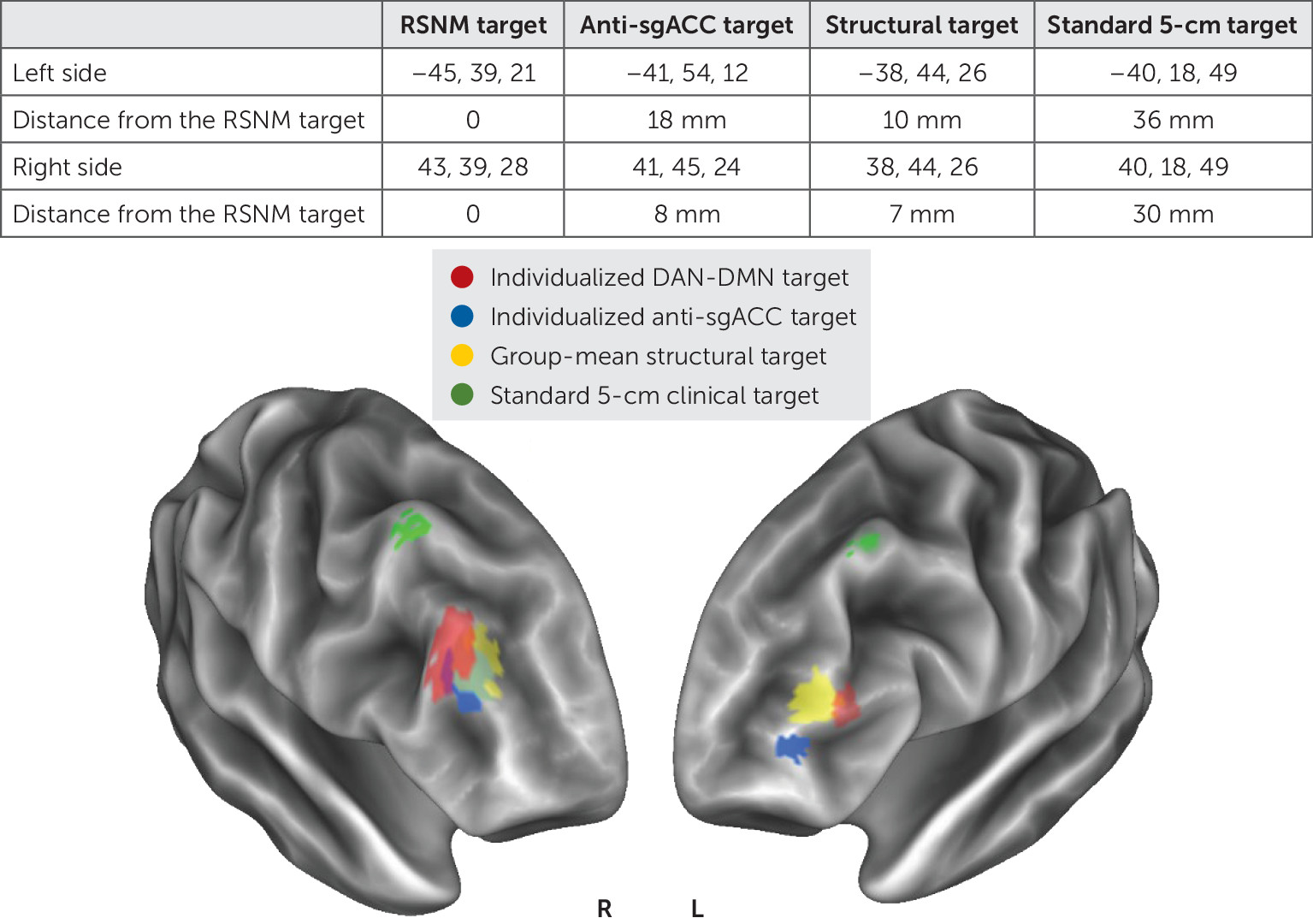
Identification of Novel Treatment Targets
The individualized map of DAN-DMN anticorrelation (
Figure 1) identified targets that were visualized on a group-mean brain surface along with targets identified by three previously reported methods (
Figure 3).
The Euclidean distance between DAN-DMN targets and the other imaging-based targets ranged between 7 mm and 18 mm (
Figure 3). The clinical “5-cm rule” target sites were at least 30 mm away on both sides. The effects of stimulation have been shown to extend approximately 12–16 mm from the stimulation site on the basis of language mapping experiments (
42) and functional connectivity analyses (
43). This suggests that the individualized targets did not overlap with targets generated by prior approaches, although it remains unclear whether this distinction is clinically relevant.
Resting-State Functional Connectivity Changes With Treatment
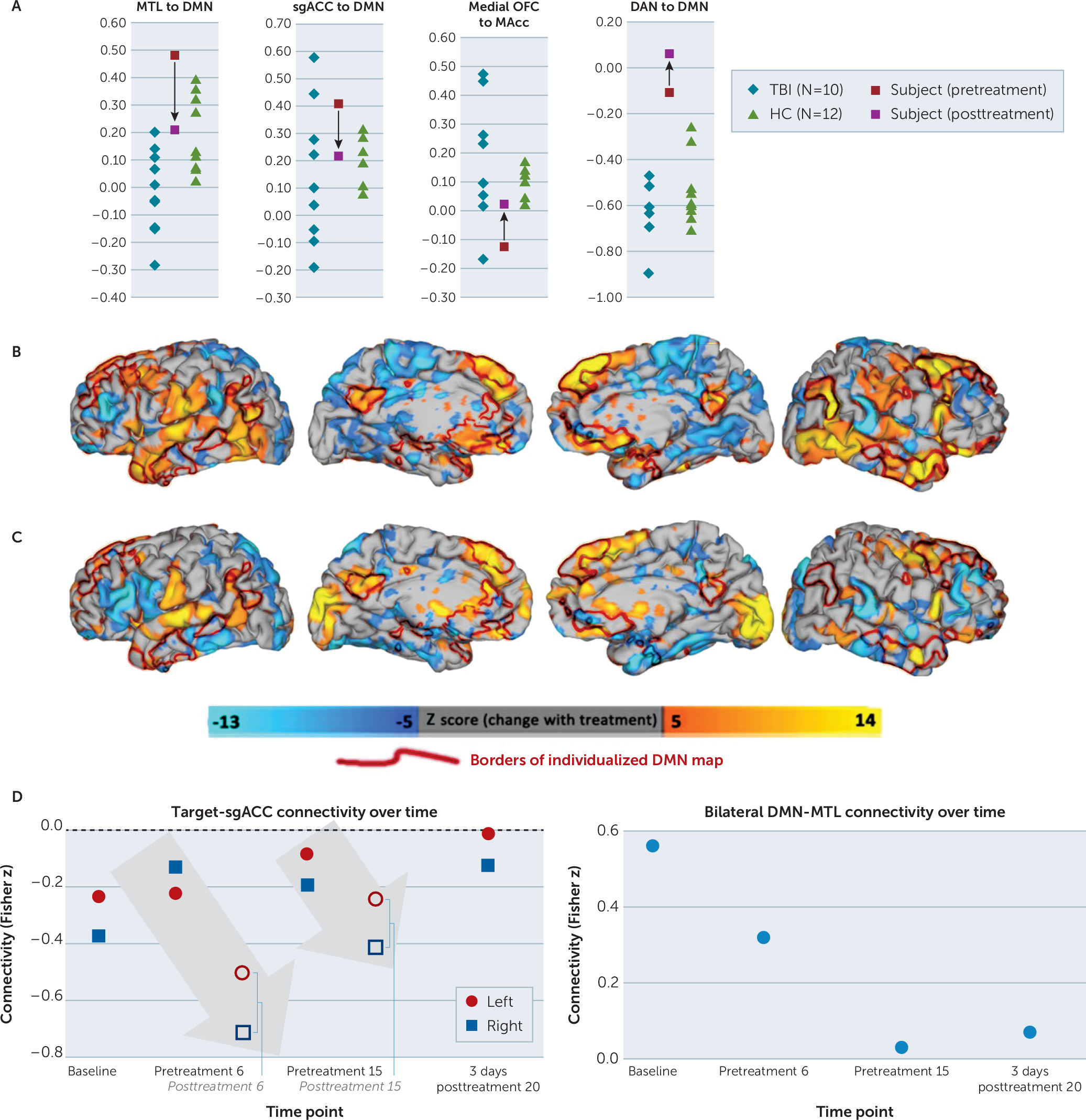
Seed-based correlation analysis suggested differences between the active subject and both comparator groups in mood-reward circuit correlations and other large-scale network correlations (
Figure 4). MTL regions showed higher correlation with DMN in comparison to healthy comparators despite lower MTL-DMN connectivity in other subjects with TBI-associated depression. Although one other subject with TBI-associated depression also showed elevated MTL-DMN connectivity, this subject was also an athlete with a history of repetitive head trauma. After treatment, MTL-DMN correlation decreased to a level that was comparable to that of healthy comparators (
Figure 4A). DAN to DMN correlation in the active subject was higher than both comparator groups and increased further with treatment (
Figure 4A). sgACC to DMN connectivity was higher than all healthy comparators and most TBI-associated depression subjects, whereas mOFC to NAcc connectivity showed the converse; after treatment, both of these values were comparable to healthy comparators (
Figure 4A, center panels). Additional exploratory comparisons are described in the supplement (for further details, see Figure S4 in the
online supplement).
Qualitatively, whole-brain seed-to-voxel connectivity maps showed that anticorrelation between the left-sided stimulation site and bilateral DMN nodes (ventromedial-dorsomedial prefrontal cortices, precuneus, and temporal poles) was attenuated with treatment (
Figure 4B). The right-sided stimulation site appeared to show a weaker pattern of anticorrelation with DMN (
Figure 4C). Treatment-induced changes in connectivity with both stimulation sites appeared to overlap closely with the subject’s individualized DMN parcellation (
Figure 4B–C), although this effect was again more prominent for the left-sided target.
Mid-Treatment Changes
Additional scans collected during the treatment course showed that the treatment successfully attenuated anticorrelation between the stimulation site and sgACC (
Figure 4C). Target-sgACC anticorrelation progressively decreased over the course of the four longitudinal time points. The acute effects of treatment, meanwhile, led to substantial increases in target-sgACC anticorrelation, suggesting that the longitudinal increase may be mediated by a compensatory homeostatic response.
Given the striking magnitude of the elevation in baseline MTL-DMN connectivity and normalization with treatment, it was unclear whether this might be attributable to regression to the mean after an erroneous initial measurement. However, post hoc analysis on mid-treatment scans revealed that the normalization of MTL-DMN connectivity occurred gradually over the treatment course and stabilized over the last few treatments (Figure S6). These mid-treatment changes may serve as a promising biomarker for early prediction of treatment response.
Discussion
To our knowledge, this is the first report of successful use of rTMS in mood disorders associated with repetitive TBI. Treatment led to improvements in clinician-assessed mood ratings, self-report emotional scores (including ratings of mood, anger, anxiety, and behavioral dyscontrol), and fluid cognition. Quantitative personality testing showed particularly drastic changes in persistence, a measure of reward-based learning that is related to mOFC-NAcc connectivity (
44) and has some predictive value for rTMS response (
45). This was accompanied by apparent normalization of connectivity in circuits related to reward-based learning and reward-motivated behavior, although this is difficult to assess conclusively in a single subject. Adverse effects were limited to transient twitching of facial muscles during treatment. Although rTMS can cause headaches (
1), the subject reported an improvement in baseline headaches.
Our successful use of individualized RSN mapping for rTMS guidance demonstrates the potential for broader clinical applications for resting-state fMRI, which are currently limited to presurgical mapping (
46). Notably, the rTMS targets identified in this subject were spatially distinct from targets identified by prior MRI-based approaches. The distance between these targets (7–18 mm) was comparable to the expected radius of stimulation with rTMS (
42,
43), suggesting there was likely minimal overlap in the stimulation volumes between different targets. All three MRI-based targets were at least 3 cm away from standard 5-cm targets, which may be partly explained by the fact that the 5-cm rule does not consider interindividual differences in head or brain size and the subject, unsurprisingly, had a larger than average head. Although this is consistent with findings that structural MRI guidance improves clinical rTMS outcomes (
47), rsfMRI-guided treatment has not yet been systematically compared with other targeting approaches. The remarkable clinical benefit in this individual warrants further investigation to answer the question of how to optimize rTMS targeting.
Although RSN mapping enables subject-specific targeting of specific functional regions, it remains unclear which region should be chosen for optimal clinical outcome. Recent literature suggests that rTMS response is related to baseline DMN correlation with sgACC (
8) as well as group-mean anticorrelation between the DLPFC treatment site and sgACC (
16). Depression in TBI is associated with dysfunction in mood-regulating circuits involving DMN and limbic connectivity with DAN (
29,
30), a system that is heavily involved in selection of stimuli based on internal expectations. Repetitive head trauma has additionally been associated with pathological and neuroimaging changes in prefrontal and medial temporal limbic regions (
34,
35,
48), which may explain the success of a structurally-oriented treatment despite failure of traditional pharmacotherapy. These early findings informed the theoretical underpinnings of our targeting approach, but further research is necessary to investigate the clinical utility of other network targets identified via individualized mapping.
The gradual changes in target-sgACC anticorrelation and MTL-DMN correlation may both serve as promising biomarkers for early prediction of treatment response. Individual stimulation sessions led to substantial increase in target-sgACC anticorrelation, whereas the longitudinal treatment course led to overall attenuation of this value; this may be mediated by a compensatory homeostatic response. DLPFC-sgACC anticorrelation is a cornerstone of many neurophysiologic models of major depression, but it has been difficult to trace longitudinally in an individual subject without individualizing the optimal DLPFC region. Similarly, MTL-DMN connectivity has been implicated in chronic traumatic encephalopathy but has also been difficult to trace individually without individualizing the DMN maps.
This study also raises questions regarding resting-state functional connectivity differences between single and repetitive TBI. The experimental subject showed notable baseline differences in comparison with both healthy comparators and individuals with single TBI-associated depression. The most striking difference appeared to be in connectivity between medial temporal lobe and the subject-specific DMN map, which demonstrated abnormalities in the opposite direction of the changes found in patients with single TBI-associated depression. The aberrant MTL connectivity observed here is consistent with emerging findings implicating MTL as a common site of pathology in chronic traumatic encephalopathy (CTE), a neurodegenerative tauopathy associated with repetitive head trauma in athletes (
34,
35,
48) Given the lack of a validated method for detection of CTE in a living person, further investigation should aim to elucidate the relationship between CTE-related tau pathology, functional connectivity, and mood disorders.
Limitations of this study include the inability to assess the effect of intensity, frequency, duration, or laterality of rTMS treatment. The use of adjunctive right-sided low-frequency stimulation, which is believed to be effective for anxiety disorders and posttraumatic stress disorder (PTSD) (
49,
50), may have contributed to the observed clinical improvement, as the neuropsychiatric sequelae of repetitive concussive TBI include a mixture of depression, anxiety, and behavioral disinhibition rather than a single categorical mood disorder. We also administered a relatively high treatment dose, which may have contributed to the subject’s improvement.
We are unable to clearly disentangle the observed clinical response from placebo effect in this individual subject. Placebo effect appears somewhat less likely given the stability of improvement upon 6-week posttreatment follow-up, although the long-term durability of treatment effects and the potential need for maintenance treatments remain unclear. Placebo effect also appears less likely given the observation of gradual changes in DLPFC-sgACC anticorrelation, which showed consistent patterns of both acute and longitudinal change. Furthermore, the gradual normalization of MTL-DMN connectivity suggests possible modulation of regions that are heavily implicated in postmortem studies of CTE.
In order to address some of these limitations, our future work will include completion of the ongoing randomized-controlled trial in a larger sample of patients with mood disorders, anxiety disorders, and PTSD associated with TBI. We are also prospectively investigating the effect of precise stimulation site on changes in network connectivity in prefrontal cortex. This lays the foundation for development of personalized neurostimulation on the basis of patient-specific brain network disturbances not only for patients with repetitive TBI and high risk for CTE but also potentially for any neuropsychiatric condition with identifiable network dysfunction.
Acknowledgments
The authors thank the experimental participant and the participants in the comparator groups. The authors also thank Drs. Eric Leuthardt and Abraham Snyder for logistical support with implementation of individualized RSN mapping; Xin Hong and Linda Hood for technical support with rTMS and MRI equipment; Drs. Sindhu Jacob and Martin Wice for referring participants; Drs. Bradley Schlaggar, Charles Zorumski, and David Soleimani-Meigooni for editorial support; Drs. Michael Fox, Steven Petersen, Charles Conway, Eric Wasserman, Sarah Lisanby, Bruce Luber, Andrew Drysdale, Irving Reti, Vani Rao, Maurizio Corbetta, Gordon Shulman, and Abraham Snyder for assistance with the conception and refinement of the study design; and Drs. Kevin Black, Pilar Cristancho, Charles Zorumski, and C. Robert Cloninger for extensive longitudinal guidance.