Role of Childhood Adversities and Recent Life Events
A recent meta-analysis of 34 case-control studies, including a total of 1,405 patients (
22), confirmed that rates of childhood sexual abuse, emotional neglect, and physical abuse are substantially higher in patients with FND, compared with healthy individuals and both neurological and psychiatric control patients. The same meta-analysis also confirmed that recent adult adverse life events are more frequent in patients with FND. The meta-analysis clearly showed that trauma and abuse are reported by some patients with FND. However, the meta-analysis also found that a substantial proportion of these patients (15%−77% across 13 studies) do not report a history of abuse, trauma, or significant life events. It is important to keep in mind that the methodology used in these studies considered only specific types of traumas, which have to be remembered and reported by patients.
It may be that some patients do not readily recall or recognize adverse life experiences; others who accurately report no history of abuse may have experienced other significant life events or stressors that are not captured by typical trauma measures or that they do not personally characterize as trauma. These events include losses of family or friends (or even significant pets for some) through deaths, divorces or deployments; bullying; illnesses; neglect; parents with substance abuse or conflict; and so on. Many times, these are remote events that occurred during developmental periods that are not recalled during symptom-focused consultations with neurologists or psychiatrists but are revealed only when a comprehensive developmental history is taken.
Several studies have investigated the links between life stressors and clinical presentation. Childhood physical abuse was found to be associated with a larger number of conversion symptoms in one study (
23), and a history of sexual abuse was linked to a different semiological presentation of psychogenic nonepileptic seizures (PNES) in another study (semiologically expressed with more emotional triggers, urinary incontinence, self-injury, nocturnal attacks, internal experiences, flashbacks, and convulsions) (
24). Likewise, a neuroimaging study demonstrated a correlation between symptom severity and childhood abuse burden and volumetric changes in the insula in women with FND (
25).
Separate from research citing trauma and abuse, other research highlights a link between physical stressors and the onset of FND. A systematic review of 133 studies encompassing 869 patients found that 37% of patients with FND reported physical events immediately prior to FND symptom onset (including injuries of the soft tissues or fractures, flu-like infectious illnesses, a neurological illness such as Bell’s palsy or migraine, acute pain, drug reactions, surgery, or syncope), increasing to 80% of patients reporting events in the 3 months before symptom onset (
26).
Evidently, antecedent stress is implicated in many cases of FND, but the nature of that stress is variable, and major life events are not an absolute criterion for the etiology, which is most likely multifactorial.
In contemporary psychological thinking, however, the nature of the stressor may be much more subtle than overt physical, sexual, or emotional abuse and often more difficult to identify with life event checklists. Indeed, using the detailed Life Events and Difficulties Schedule (LEDS) (
27), a study found that significant stressors were identifiable in 91% of patients with FND, even though 88% of these events were not mentioned in the clinical notes, suggesting that they were not seen as relevant by the referring physician or not mentioned by the patient (
28). One caveat of this study was that it included patients from a particular area of London with lower socioeconomical resources and may not be representative of all FND patients. This study also found that 53% of patients with FND had, according to blind raters, experienced life events from which physical symptoms might offer some kind of escape, compared with only 13% of a depressed control group. Such a finding is difficult to explain from a purely cognitive or biological perspective and points to a role for conversion processes in at least a proportion of patients with FND. However, it is important to note that although psychosocial stressors may be present in the majority of FND cases, specific stressors, such as remote or recent trauma, are likely to affect some but not all of the neurobiological processes underlying FND.
Repression of Adverse Memories in FND
According to the conversion model, a key function of the defenses is to prevent the recall of memories associated with the distressing events in question. Neuropsychology research has investigated this phenomenon of memory suppression and verified whether it is possible to voluntarily and actively forget unwanted memories with a think–no-think task (
29). In this paradigm, subjects have to learn pairs of words (such as ordeal-roach or steam-train). Participants are then asked to either remember (think) the second word (roach) when presented with a cue (ordeal) or try not to remember (no think) the second word (train) when presented with the cue (steam). Finally, they are asked to recall all of the word pairs. Evidence showing that the think pairs (ordeal-roach) are better remembered than the no-think pairs (steam-train) supports the view that there are cognitive processes that allow us to forget some memories. A subsequent study looked at which regions of the brain were activating during this process, and a network involving increased dorsolateral prefrontal cortex (DLPFC) and decreased hippocampus activity was found (
30).
With the aim of studying this network in FND, an fMRI task compared active recall of autobiographic memories in FND patients and healthy controls (
31). First, a LEDS interview (as discussed above) was conducted to identify stressful memories. Then a panel of experts, blinded to whether a patient or a healthy control was being assessed, judged the severity of the event (0–4 score) as well as the escape/secondary gain that could theoretically be obtained if a physical conversion/functional symptom followed the traumatic event. This allowed two kinds of severe events to be classified: escape events (with secondary gain) and severe events (without secondary gain). Patients were also asked to subjectively rate the severity of these events. Even though they were of equal objective severity (as assessed by the blinded panel), the escape events were perceived as significantly less severe by FND patients. This is compatible with the conversion theory, which suggests that the primary gain of alleviating the emotional load attached to the autobiographical memory worked for this particular type of events.
The two regions identified as relevant in the suppression of unwanted memories (DLPFC and hippocampus as discussed above) were then chosen as a priori regions of interest for the fMRI recall task (
31) (see Figure S1 in the
online supplement). The recall of escape events elicited increased DLPFC and decreased hippocampal activity in FND patients (but not in healthy controls), suggesting the suppression of unwanted memories in this group. It seems that some traumatic autobiographical events are indeed processed differently in the brains of FND patients, consistent with the conversion model.
Neural Correlates of the Conversion Mechanism
One of the challenges of the conversion model has been “measurement” of the unconscious. In the past, we have had to look at the issue indirectly. Clinically, the unconscious has been “tapped” and identified through decades of hypnosis research (
32–
34). With functional neuroimaging, a third result from the above-mentioned fMRI task investigating recall of traumatic life events (
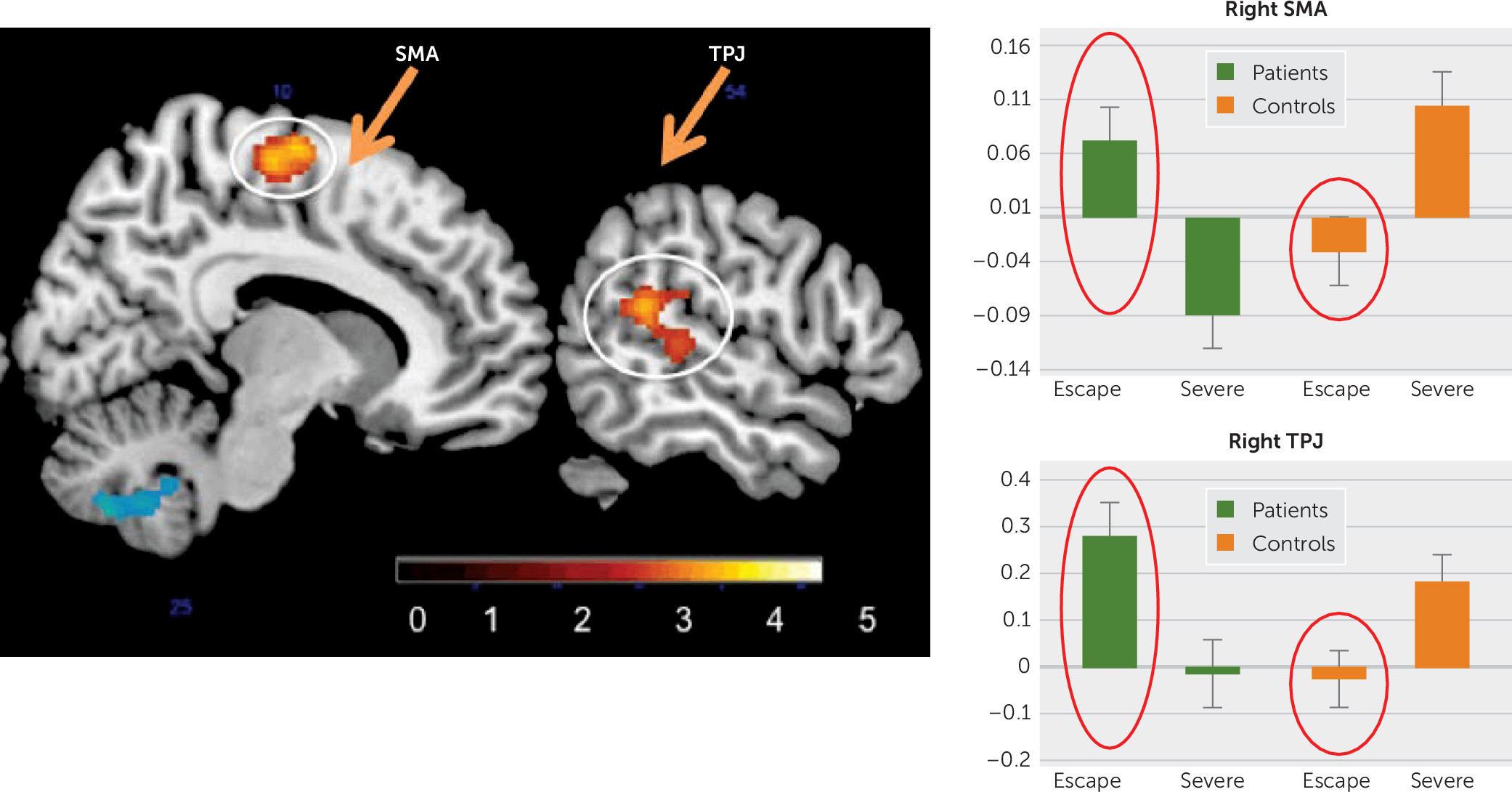
31) is directly related to a possible conversion mechanism. In the whole-brain analysis, compared with controls, FND patients had a significant increase of activity in a cluster that included the right supplementary motor area (SMA) and another that included the right temporoparietal junction (TPJ) when they were recalling escape events more than when they were recalling other severe events (
Figure 2). These are important findings for three reasons. First, the task was not targeting motor or sensory function, and yet it elicited peak responses in the SMA and the TPJ (two regions relevant in the production of physical FND symptoms). Second, this pattern was not found in all events but only in the ones with escape–secondary gain potential. Third, this was specific to FND patients and not found in healthy controls. These findings are compatible with the theory of conversion that some memories may influence brain regions implicated in sensorimotor (neurological) symptoms. The SMA is central in motor execution and inhibition (
35) of conscious (
36) and unconscious (
37) prepotent responses. The TPJ is a key region implicated in multisensory integration, in the sense of self-agency and in making sensorimotor predictions (
12). Both the SMA and the TPJ, among other networks, are key in FND symptom production, as shown by the mechanistic line of research (
10,
11,
38,
39).
Protection Against a Threat
Other research suggests that patients with FND respond differently to psychological stressors, compared with healthy controls. For example, an experiment studied patients with nonepileptic seizures using an emotional Stroop test where participants had to name colors on which subliminal emotional faces (happy or angry) were superimposed (
40). It was found that FND patients took significantly longer than healthy controls to name colors superimposed on angry faces, suggesting that the threat value of the faces was drawing attention away from the primary task in this group, even though they were not accessible to consciousness. Consistent with this, another study found that children and adolescents with FND were disproportionately quicker to identify sad faces than happy faces, compared with healthy controls, suggesting that the FND group was more efficient when the task was to identify social threat cues (
41). A related finding was obtained in a study comparing healthy controls to patients with motor FND (
42). In this study, participants had to maintain a constant force by pinching a force-measuring device between their thumb and index finger over 6 seconds when viewing pictures with or without threatening content (e.g., a snake or gun). Although healthy controls showed a decay in force over time for both picture types, the FND group maintained an abnormally high force in response to the threatening pictures, also suggesting altered threat processing in this group.
Animal defense mechanisms.
Some commentators have proposed examining animal threat response behaviors as a way of understanding bodily reactions to threat in patients with FND (
43). Traditionally, fight-or-flight models have been used to describe both animal and human behavior under stress (
44). However, sometimes neither fight nor flight are adequate or acceptable (e.g., in the case of social threat), which may explain the existence of other protective mechanisms, such as tonic immobility (
45) and distraction displays (
46,
47).
One possibility is that the symptoms of FND originate in innate defensive behaviors and manifest in response to adverse developmental or other experiences (
48). Three examples of defensive behavior present in animals are sometimes cited as potential representations of animal models of FND.
Distraction displays are one example of defensive behavior in animals. One animal model often cited with reference to FND is the distraction displays observed in some birds (killdeers and plovers) protecting their nests. For example, killdeers (
Charadrius vociferous) display “injury-feigning” or “broken-wing” behavior in the absence of real injury (
46,
47) as a way of distracting the attention of predators when they approach the nest. This is meant to attract the predator away to a safe distance from the nest, after which the bird can escape. However, videos of the killdeer show the bird looking around to see whether the predator threat is clear before resuming normal function, suggesting some degree of intentionality (
49). Therefore, we suggest caution before interpreting a distraction display as akin to the unconscious defensive process postulated in the conversion model of FND.
Tonic immobility—also known as thanatosis—is another antipredator behavior adopted by prey, which occurs late in an attack sequence when the fight behavior has failed and the escape possibilities are gone (
50). This is a last-resort option (
51), displaying motor inhibition, hypertonicity, and relative unresponsiveness (
52)—mimicking death in order to avoid being eaten. In studies of opossums threatened by human or dog vigorous tactile stimulation, reductions of heart rate, respiratory rate, and body temperature occurred during death feigning, despite the animal’s being fully conscious (
53). Although this behavior may resemble the atonic dialeptic semiology of nonepileptic seizures in humans, it is unclear whether there are other similarities between this phenomenon and FND in humans.
Freezing is an unlearned defensive response to threat, present in many species, including cats, whose main features are immobility, increased muscle tonus, and bradycardia (
54). In contrast to tonic immobility, which is characterized by a relative unresponsiveness and occurs late in the threat sequence as a last-resort behavior, freezing is thought to confer survival advantages by avoiding detection by the predator, enhancing perception and attention and preparing the animal for escape (
54). Freezing also exists in humans (
55), and its study in FND patients will be discussed below.
Conversion disorder in humans: the relevance of metacognition and rerepresentation.
Unlike the animal behaviors noted above, FND symptom formation in humans can arise in the absence of a physical, visible, immediate threat. It can be hypothesized that a remote stressor, such as a memory-reminiscence of an actual or imagined threat can have similar psychobiological consequences. This would be in line with the imaging study described above (
31), showing that a traumatic memory can induce a “short-circuit” in the brain and interact with normal motor and sensory function (
Figure 2). Whether this short-circuit uses the same mechanisms as the one discussed in animals still needs to be established.
One obvious question when it comes to comparing animal and human behavior is the role of more advanced forms of cognition found only in humans, such as metacognition (i.e., awareness and understanding of one's own thought processes, or “thinking about one’s thinking”) and re-representation (i.e., “systematic transformation of the psychological encoding of a stimulus [e.g., scene, situation, object, narrative] from an initial set of elements representing the meaning of the stimulus to a subsequent, different set of elements,” or “the action of representing a person or thing again” [
56]). Although some comparative psychologists argue that these processes are uniquely human characteristics (
57), others suggest that dolphins and chimpanzees, for example, have analogs of human metacognition (
58). Rerepresentation allows humans to reflect beyond the self-evident story (e.g., immediately observable life-threatening stimulus) to multilayered narratives, and metacognition allows them to be aware of their own thinking.
A psychological model of conversion (FND) could be that both of these higher-order cognitive processes are cognitively required to be able to develop FND—i.e., both are present in (and possibly unique to) humans—and that these processes are disordered in patients with FND. This may explain how an inner thought or memory or intrapsychic conflict could have an effect similar to an external threat, and we propose that both of these higher-order cognitive processes (
59) play a role in the development of FND.
Neural correlates of defense behavior.
There is evidence to suggest that the neural correlates of threat processing in the limbic system, and particularly the amygdala, are different in patients with FND, compared with individuals without FND. For example, an incidental affective task was conducted in motor FND patients and healthy controls undergoing fMRI, in which participants had to determine the gender of neutral, angry, or happy faces (
60). Although healthy controls showed only increased amygdala activation to angry faces, compared with neutral faces, the FND group showed this effect for both angry and happy faces. This suggests that FND patients have a hypersensitivity to emotional stimuli in general, perhaps suggesting that all social cues are perceived as potentially threatening.
A similar effect was seen in a study that found increased activity in the left amygdala in FND patients, compared with heathy controls, in response to both sad and angry faces (
61). In this study, the left amygdala also became more activated over time in the FND group in response to fearful faces, compared with sad faces, seemingly suggesting sensitization, rather than habituation, to the fearful stimuli. This study also found that patients with motor FND had greater activity than controls in the periaqueductal gray area (PAG) when processing negatively valanced emotional stimuli (both sad and fearful faces) (
61). This region of the brainstem is key in the network underlying the freeze response, as shown both in animal research (
62,
63) and in studies of humans (
64).
Another recent study found changes in brain connectivity between the amygdala and the PAG in FND patients (
65). To test whether patients with FND exhibited an abnormal freeze response, a behavioral study measured postural changes during the Trier social stress test, in which participants are asked to simulate a job interview in front of two examiners (
66). In this study, healthy controls showed a reduction of movements of the trunk in the second part of the interview (suggesting a normal freeze response), whereas patients with FND failed to show this behavior. These are preliminary data from a small sample and did not include measures of bradycardia, which would have helped in making inferences about the complete freeze response. What is suggested, though, is that patients have an abnormal postural response to stress. This finding cannot be generalized to all subtypes of FND, because even though there are dialeptic (motionless unresponsiveness) semiological presentations of PNES, most seizures and motor symptoms are hyperkinetic, not akinetic. More research is needed to elucidate why some patients have hyperkinetic and other patients have hypokinetic responses in FND.
In summary, there is currently convincing evidence that FND patients have abnormal threat processing resulting in a measurable abnormal body response. If a parallel animal body reaction to threat existed, it could argue for the “innate” nature of the conversion-body response. On the other hand, arguments exist for an “acquired” process, in which events and experiences become threatening because of the personal meaning they have for the individual in the context of his or her specific learning history. At this stage, more research is needed, perhaps taking an interdisciplinary approach with ethologists and sociologists to bring a fresh perspective on how and why an emotion or a painful thought can be transformed into a physical symptom.
Biological Stress and Stressors
Stressors are not the same as stress. Some patients experience events and symptoms when not stressed. Evidence, however, for an elevated stress response in patients with FND has been found in biomarker studies. In a study using the emotional Stroop test, for example, the authors found a significant positive correlation between FND participants’ reaction times to angry (i.e., threatening) faces and their basal cortisol level (
67). This effect was confirmed by findings of elevated diurnal basal salivary cortisol levels in FND patients with nonepileptic seizures (
68) and those with motor FND (
69). The latter study also found increased salivary amylase levels (a protein secreted in response to adrenergic activation) in FND patients, compared with controls. This implicates both the hypothalamic-pituitary-adrenal axis (which is responsible for heightened cortisol levels) and a second stress pathway involving rapid adrenergic responses in FND (heightened amylase levels). Other evidence of abnormal biological stress regulation in FND comes from studies of the autonomic system. Impaired vagal tone, as measured by several heart rate variability indices, has been found in both adults (
70,
71) and children (
72) with FND.
Whether abnormal biological stress regulation plays a causal role in FND or is secondary to the disorder cannot be resolved yet, and further studies are needed. An association with childhood adversities has been found: basal cortisol levels were higher in FND patients, compared with controls, and even higher in a subgroup of FND patients who experienced childhood sexual abuse (
68). A correlation between the number and impact of life events and basal cortisol levels was also found in a group of FND patients but not in healthy controls (
69). This suggests a link between life stressors and biological measures that is specific to patients with FND. A possible hypothesis is that FND patients have a vulnerability to adverse events, which leads to abnormal stress regulation at both a biological and a neural level. Further research on these links might better explain why and how a stressor can be linked to a physical symptom as implied by the conversion model.
At present, there is a lack of evidence concerning the role of genetic factors in FND, with only a small number of familial cases having been published (
73). There might, however, be a genetic vulnerability that predisposes some individuals to react to the environment—and in particular to stressors—in a different way, leading to FND symptoms. A preliminary study identified increased methylation rates on the promotor of the oxytocin receptor gene in a group of mixed FND patients (
74). The gene is implicated in stress regulation, and broader studies testing several genes might shed light on the gene-environment interactions in FND.