The cholinergic system plays an important role in human cognition. Evidence of degeneration of this system has been reported by postmortem and in vivo imaging studies in neurodegenerative disorders, especially Alzheimer’s disease (AD) and dementia with Lewy bodies (DLB).
The cerebral cholinergic system that is involved in the regulation of attention and higher order cognitive processing is grouped into eight distinct nuclear groups (cholinergic cell groups Ch1–Ch8) based on the efferent projection to their anatomical targets. Efferents from the Ch1 (medial septal nucleus) and Ch2 (vertical limb of the diagonal band of Broca) provide major innervation for the hippocampus via the fornix; Ch3 (horizontal limb of the diagonal band of Broca) provides innervation for the olfactory bulb; Ch4 (nucleus basalis of Meynert) delivers major cholinergic input to the neocortical mantle and amygdala; and Ch5–6 (pedunculopontine and laterodorsal tegmental nucleus) provide cholinergic projections to the thalamus, basal ganglia, basal forebrain, other brainstem structures, and spinal cord (
1–
4). Efferents from Ch7 (medial habenula) and Ch8 (parabigeminal nucleus) provide innervation for the interpeduncular nucleus and superior colliculus, respectively (
3). Because these systems support a number of cognitive, neurobehavioral, and motor functions, cholinergic losses are thought to play an important role in cognitive and neurobehavioral changes in AD, Parkinson’s disease (PD) and DLB (
5–
8). We have previously shown that cortical (Ch4) cholinergic losses were more severe in DLB and PD dementia compared with AD and PD without dementia (
9), and they were correlated with the degree of cognitive impairment (
7). It is plausible that the earlier manifestation of neurobehavioral symptoms in DLB compared with AD may, in part, reflect more severe or more extensive cholinergic losses that are not limited to cortical changes but also involve subcortical changes (
1).
Characterizing the severity and topographic extent of cholinergic denervation in DLB may provide better insight in the relationship between cholinergic system changes and clinical manifestations, such as cognitive fluctuations, attentional deficits, visuospatial and visuoperceptual changes, visual hallucinations, or falls in DLB (
10,
11). In vivo brain imaging studies of DLB have shown nigrostriatal dopaminergic and more diffuse brain cholinergic losses (
12). However, a limitation of previous cholinergic imaging studies in DLB was the use of either global cortical or large lobar volumes of interest to characterize cholinergic innervation changes in this disorder. This may have resulted in missed recognition of small-sized regional cholinergic changes due to dilution when computing average brain binding changes in large lobar or global cortical volumes of interest. The purpose of the present study was to explore a more granular topographic assessment of regional cholinergic binding differences between DLB and control subjects using a spatially nonbiased whole brain voxel-based analysis of vesicular acetylcholine transporter (VAChT) [
18F]-fluoroethoxybenzovesamicol ([
18F]-FEOBV) positron emission tomography (PET). We hypothesized that cholinergic brain changes do not follow a globally diffuse denervation pattern but that cholinergic denervation changes in DLB may depend on specific regional brain functions. Here, we report the findings of [
18F]-FEOBV PET obtained from five participants with DLB and 21 healthy control subjects. [
18F]-FEOBV whole brain and volume-of-interest PET data from a subset of these participants have been previously reported (
1).
Participants
The patients were recruited at the Cognitive Disorders Clinic in the University of Michigan Health system. Third International Consensus criteria were used to diagnose probable DLB (
13). The control group was acquired from our poll of the existing normal control elderly PET database matched with our patients’ age and gender. At the time of the PET scans, the normal controls had a normal neurological examination with no history of neurological or psychiatric diseases. Any participants who had evidence of large vessel strokes or other intracranial lesions were excluded from the current study. Written informed consent (or assent) was collected from the participant (or legal representative) before study participation. The study was approved by the University of Michigan Medical Institutional Review Board and in compliance with the Declaration of Helsinki guidelines. All participants underwent brain MRIs and delayed acquisition (3–3.5 hours, scanned every 5 minutes for a total of six frames) [
18F]-FEOBV (bolus intravenous of 8 mCi) PET. T
1-weighted imaging was performed on a 3-T Philips Achieva system (Philips, Best, the Netherlands). A three‐dimensional inversion recovery‐prepared turbo field echo was performed in the sagittal plane (repetition time/echo time/inversion time=9.8/4.6/1,041 ms; turbo factor=200; single average; field of view=240×200×160 mm; acquired matrix=240×200×160 slices) and reconstructed to 1‐mm isotropic resolution. PET imaging was performed in three-dimensional imaging mode with a Siemens ECAT Exact HR+tomograph, as previously reported (
14). [
18F]-FEOBV was prepared in high radiochemical purity (>95%) (
15). Delayed dynamic imaging was performed over 30 minutes (in six 5-minute frames) starting 3 hours after an intravenous bolus dose injection of 8 mCi [
18F]-FEOBV (
3). The PET imaging frames were spatially coregistered within subjects with a rigid-body transformation to reduce the effects of subject motion during the imaging session.
Results
This cross-sectional study consisted of five participants with DLB (females, N=3; males, N=2), with an average age of 77.8 years (SD=4.2), an average disease duration of 4.5 years (SD=2.1; median=6; range=4), and a mean Mini-Mental State Examination (MMSE) (
17) score of 18.6 (SD=4.8). The core clinical criteria of the participants with DLB are presented in
Table 1.
Demographic and clinical characteristics of the DLB and control groups are presented in
Table 2. Twenty-one elderly healthy control subjects (females, N=13; males, N=8) were examined, with an average age of 73.62 years (SD=8.37) years and a mean MMSE score of 28.67 (SD=1.35).
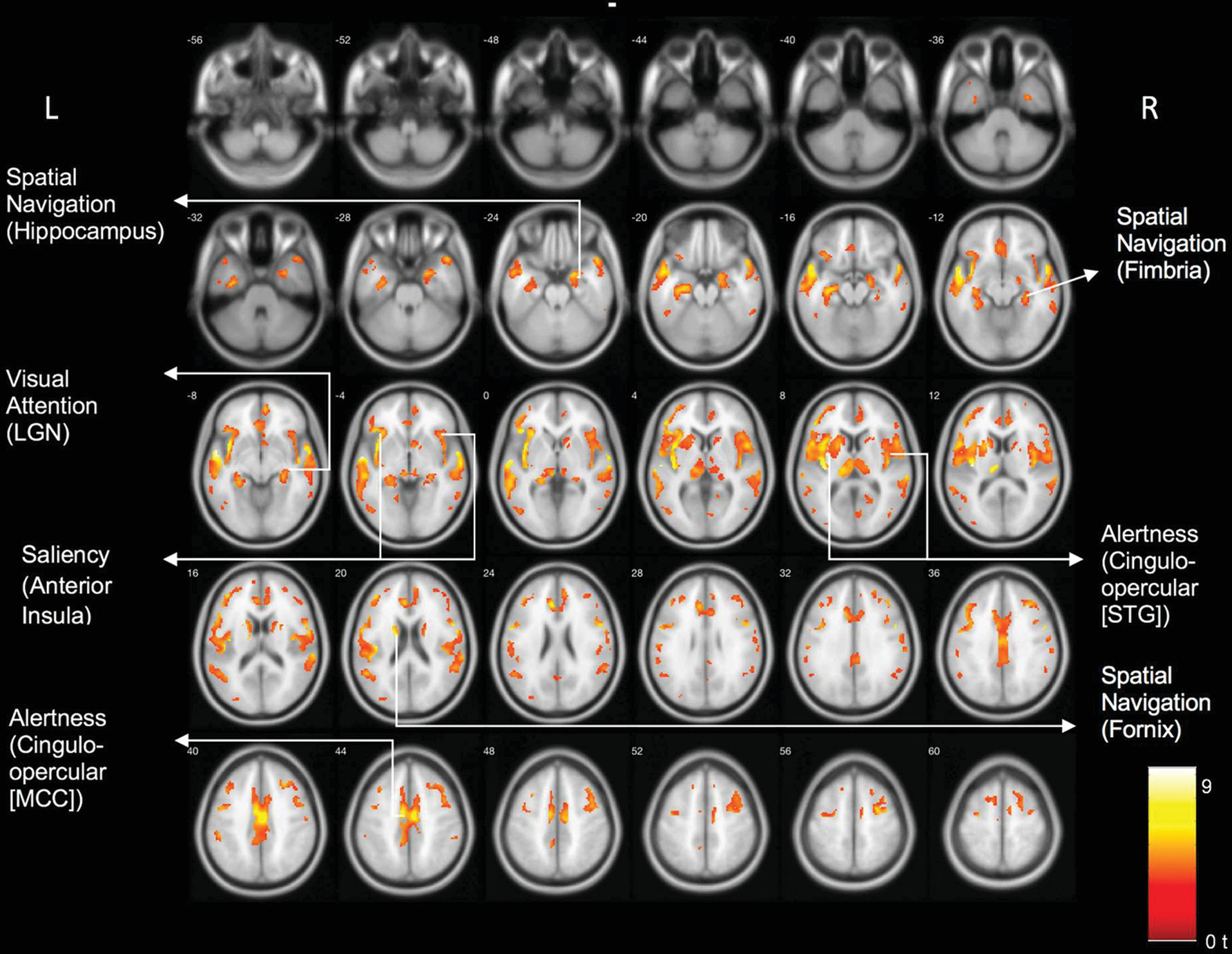
Results of the voxel-based comparison between the DLB and control group are shown in
Figure 1. There were significant cholinergic binding reductions in the DLB compared with the control group in the following areas: the bilateral opercula, anterior to mid-cingulate cortices, bilateral insula, right more than left lateral geniculate nuclei, pulvinar, right proximal optic radiation, bilateral anterior and superior thalami, and bilateral posterior hippocampal fimbria and fornices.
Table 3 lists the main clusters and their peak MNI coordinates and associated network hubs. We repeated this analysis with years of education included as a covariate. This analysis yielded similar results (see Figure S1 in the
online supplement).
Exploratory Post Hoc Cognitive Correlation Analysis
We performed an exploratory voxel-based regression analysis of the PET data and the MMSE as an outcome parameter within the group of the DLB participants. Results are shown in Figure S2 in the online supplement. Clusters (uncorrected p<0.05) were seen in the following regions: bilateral lateral geniculate nucleus, bilateral lingual gyri/optic radiations, left medial occipital, left superior posterior parietal, right superior parietal, right posterior frontal, right superior temporal cortices, and the right hippocampus. No clusters meeting this criterion were found in a similar voxel-based PET-MMSE regression analysis in the normal control group.
Discussion
Voxel-based whole brain analysis of VAChT PET identified a distinct topographic cholinergic denervation pattern rather than diffuse cerebral losses in DLB compared with the elderly control group. Regions showing cholinergic denervation overlapped with key hubs of neural networks responsible for visual attention (visual thalamus) (
18), salience (insula) (
19), spatial navigation (fimbria/fornix) (
20), and maintenance of alertness (cingulo-opercular) networks (
21). Specifically, we found cholinergic changes in the following hubs of each network: maintenance of alertness (cingulo-opercular) network: insula, operculum, cingulum, thalamus; salience network: anterior insula, anterior cingulum; visual attention network: visual thalamus, including the lateral geniculate nucleus; spatial navigation network: fornix, fimbria, hippocampus, and anterior thalamus.
Graph theory analysis of functional connectivity resting state MRI identified two distinct top-down task-control networks (
22,
23). The dorsolateral prefrontal cortex and intraparietal sulcus are hubs within the frontoparietal task control (FPTC) network. The FPTC network is important for start-cue and error-related activity and may initiate and adapt control on a more rapid and trial-by-trial basis. The cingulo-opercular task control (COTC) network encompasses medial superior frontal cortex, dorsal anterior cingulate, frontal operculum, anterior insula, and thalamic regions (
22). Effectively, the COTC graph may include parts of both the large-scale distributed cingulo-opercular network and the salience networks (
23). The topography of cholinergic reductions in our patients with DLB suggests preferential cholinergic involvement of the COTC. The COTC network controls the goal-directed behavior more on a set maintenance mode using a longer duration time scale, such as needed for maintaining alertness. Clinically, this may be reflected in a lack of maintenance of a functional operative state and difficulties staying on task in persons with DLB in their daily life functions, resulting in cognitive fluctuations. Our findings are also compatible with the emerging notion of an important role of the cingulo-opercular network in task-switching from a passive task to an active task (
24).
Another interesting observation was the asymmetry of visual thalamic region findings, such as right more than left lateral geniculate nuclei, pulvinar, and right proximal optic radiation changes. These findings may reflect associations with the ventral attention network, which has a right-hemispheric dominance (
25). The ventral attention network is involved in the detection of bottom-up salient and behaviorally relevant stimuli in the environment, especially when the stimuli are initially unattended. This network is highly integrated with the visual system and includes the ventral part of the supramarginal gyrus, ventral frontal cortex, posterior part of the superior temporal sulcus and gyrus, inferior frontal gyrus, middle frontal gyrus, frontal operculum, and anterior insula, which acts as a “network breaker” that interrupts attention in the dorsal system and reorients attention toward a stimulus-driven object (
26). These observations agree with our previous findings that lower cholinergic activity in the thalamus was associated with decreased saliency processing in patients with PD (
27). This is likely mediated by the right lateral geniculate nucleus, where decreased cholinergic innervation may be associated with compromised visual attention, perceptual abnormalities, and possibly hallucinations in patients with DLB. We also recently reported on an association between reduced cholinergic integrity of the right lateral geniculate nucleus and falls in patients with PD (
28). A similar association may exist in patients with DLB; however, this will require further confirmation.
The hippocampus plays an important role in spatial navigation (
20), which is a key function for safely walking. Our findings show evidence of reduced cholinergic transporter binding in the hippocampal fimbria and fornices. Cholinergic deficits in these regions may compromise the integration of cognitive and sensorimotor functions while walking. Clinically, these regional deficits may hypothetically be associated with wandering behavior in the patients with DLB.
Exploratory findings of a voxel-based whole brain regression analysis of the cholinergic PET data, using the MMSE as the outcome parameter, showed several clusters that overlapped with several of the topographic cholinergic denervation regions. A notable finding was involvement of the visual system, especially the bilateral lateral geniculate nuclei and adjacent visual lobe regions. This may possibly be associated with visual processing dependent cognitive and/or neurobehavioral changes in DLB. Other regions showed overlap with the COTC and spatial navigation networks. No clusters meeting this criterion were found for a similar analysis in the normal control group, suggesting disease-specific cognitive correlates in the DLB group. These preliminary findings need to be confirmed in a larger sample size.
Identification of neural network changes underlying the clinical symptom manifestations in patients with DLB would augur network neurostimulation approaches as a complementary treatment strategy to existing pharmacological and behavioral interventions. Furthermore, our study has identified hubs of key neural networks that may contribute to the clinical symptom manifestation of DLB. Future studies comparing in vivo imaging findings directly with postmortem data may be very informative to advance our understanding of the neurodegenerative mechanisms underlying DLB. For example, findings of cholinergic changes in the lateral geniculate nucleus in DLB are very novel, but at present there is a critical gap of knowledge to explain the cholinergic vulnerability of the lateral geniculate nucleus in DLB. Another topic of future in vivo-ex vivo correlation research would be to assess for pathological changes in hubs corresponding to regional network vulnerability in postmortem data.
There are several limitations of this study. One limitation is the small sample size. Further studies using a larger sample sizes are needed to validate these topographic VAChT findings and associate them with clinical symptoms. The second limitation is the cross-sectional design, which lacks longitudinal information about the cholinergic binding areas and how cholinergic vesicular transporters may change in relationship to the temporal manifestation of specific clinical symptoms. Third, we do not have detailed neuropsychological testing data to validate the clinical diagnosis of probable DLB. Lastly, our neural network interpretation of the topography of neuroimaging data in the absence of neuropsychological metrics, functional MRI, or electrophysiological measures remains speculative.