Neuropsychiatric disorders include mental and behavioral disorders as well as neurological disorders, which are responsible for 10.4% of total global disability-adjusted life years (
1). World Health Organization research has shown that there are large inequalities in access to neuropsychiatric care across different populations. There is a global shortage of neurologists (
2), psychiatrists (
3), and other health care professionals who treat these conditions, and there is a need for innovative strategies to help patients with these disabling neuropsychiatric conditions. Many companies and individuals have developed smartphone applications (apps) that claim to help these patients. In 2019, it was estimated that 2.7 billion people would own smartphones and have access to more than 325,000 mobile health (mHealth) apps (
4,
5). While there is a negative association between age and the use of smartphone apps (
6), research suggests that the rise and use of these apps among neuropsychiatric patients is substantial (
7). With the rapid growth of these monitoring and self-management tools, there is great potential for collecting and sharing personal and health-related information (
8).
Methods
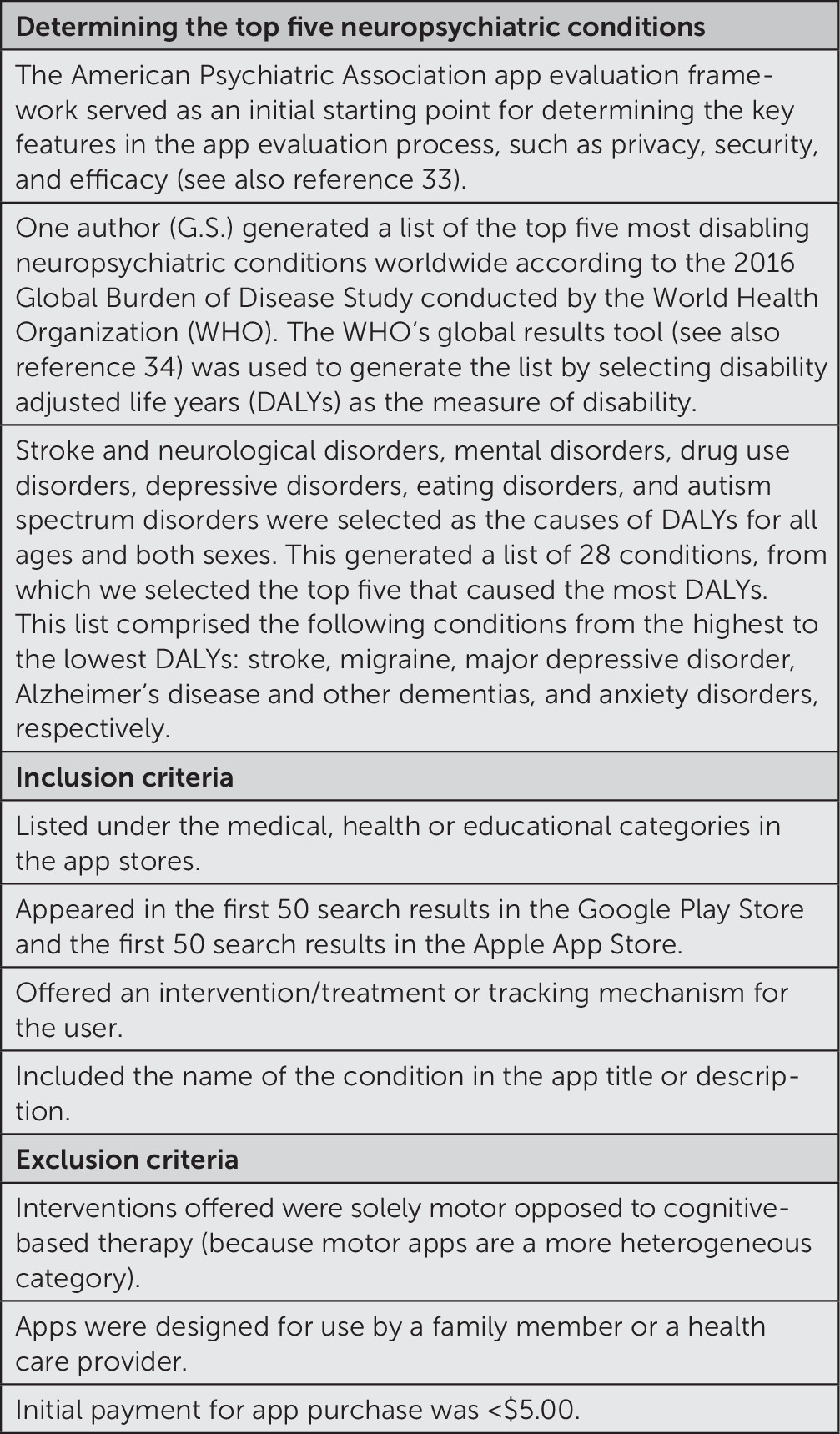
Figure 1 shows the method for determining the top five neuropsychiatric conditions (stroke, migraine, depression, Alzheimer’s disease and dementia, and anxiety) and the method of selection of apps for inclusion or exclusion from the study as determined by the team of experts on the author panel. By using the list of the top five neuropsychiatric conditions, a systematic search of the Google Play and Apple app stores was conducted between July 30, 2018, and September 2, 2018. By using these search results, two authors (A.G. and G.S.) selected and evaluated the top 15 apps in each operating system’s store that met criteria corresponding to the following search terms: stroke/stroke therapy, migraine, depression, Alzheimer’s disease and dementia, and anxiety. Many of the stroke apps that appeared in the initial search were ineligible, as they were typically designed to help clinicians or family members determine if someone was having a stroke. For this reason, the search term stroke therapy was then used as opposed to stroke. If an app appeared in both of these stores, the app was only counted once. These apps were cross-referenced between the same two authors (A.G. and G.S.) to determine whether there were any differences in functionality or features offered in different operating systems. Of note, as we were conducting the search, the order of apps appearing in the search results from both app stores varied on a daily basis, related to Apple and Google’s proprietary rankings and featuring of apps. One author (A.G.) installed apps on an Android device and abstracted data to review the operation of Google ecosystem apps. Similarly, G.S. installed apps on an iOS device and abstracted data to review the operation of Apple ecosystem apps. The mobile health apps reviewed on the Google Play and Apple app stores were all publicly accessible. Patients were not involved in this study.
Data Collection
Between July 30, 2018 and September 2, 2018, the two authors (A.G. and G.S.) abstracted data from the apps, the app stores, and the app developers’ websites. Data extracted included the following details: app ratings and search logistics, target population, app function, the existence of a privacy policy, intercommunicability, and app/developer endorsements. Details of the search, the data abstracted, and the definitions developed by coders for each of the features are presented in Tables S1 and S2 in the online supplement. The research team did not attempt to evaluate the technical functioning of the apps or seek to corroborate the claims of the app developer. As noted in the table, apps were included in the study only if they were free or if the initial price for app purchase was <$5.00.
When apps were available for free, the team did not pay for subscriptions, premium versions, or the full versions of Lite apps. Initial purchases were made for the paid versions of one of the Android apps and seven iOS apps. The research team evaluated the app websites for information pertaining to prior research studies conducted using the app (in the form of meeting abstracts and publications) to determine whether the intervention components of the apps were deemed evidence based. We did not conduct our own independent review outside of the app websites to determine whether the apps were used in research studies. The research team also abstracted the app websites for information pertaining to endorsements from other organizations. We recorded whether there was a privacy policy on the websites, in the apps themselves, or in the Google Play Store and Apple App Store. Even one-sentence privacy policies were included in this study. Given the differences between Google and Apple products, some modifications in the searches were required. Specifically, the Apple App Store did not provide information on number of app downloads per app, but the Google Play Store did.
Data Analysis
Data were stored in Excel, and descriptive analyses are reported. Initially, we planned to have an attorney who was a certified information privacy professional (CIPP) review the privacy policies and data abstracted from them for accuracy in order to ensure that the two authors (A.G. and G.S.) could reliably abstract and interpret the privacy data and assess whether typical users would be able to understand the content of the privacy policies.
Because the 83 apps examined were initially reviewed from July 30, 2018 through September 2, 2018, we also sought to determine whether they were currently available for download in an app store and whether they were active and still being used by the general public. Apps were considered active if they had a user review dated September 1, 2019, or later. Thus, after submission to the journal and prior to publication, we rereviewed the apps to ensure that the data we presented were still relevant.
The study was exempt from IRB approval, as all data collected were publicly accessible and no human subjects were included.
Results
Five neuropsychiatric conditions (stroke, migraine, depression, Alzheimer’s disease and dementia, and anxiety) were considered in this study, and a maximum of 15 apps per condition could be selected from each app store. Some apps were available in both app stores; in addition, after conducting the search we realized that some apps were listed under both anxiety and depression. Thus, a total of 83 apps were reviewed (stroke, N=8; migraine, N=25; Alzheimer’s disease and dementia, N=8; depression, N=7; anxiety, N=14; anxiety plus depression, N=21). Details regarding the specifics of the apps evaluated for each condition can be found in Tables S3–S8 in the
online supplement. Downloaded data for each category of apps are summarized in
Table 1. Mean app downloads were highest for anxiety (over 2.12 million) followed by anxiety plus depression (135,769) and then migraine (72,440). All of the apps were reported to be self-help apps. The vast majority of all of the apps (with the exception of migraine) included an intervention component (69%). However, less than a quarter of the migraine apps were considered to include interventions (24%), although 76% of the apps for migraine were for tracking data. Apps for anxiety and apps for anxiety plus depression were also for tracking, 50% and 57% respectively. Very few of the apps that provided interventions were considered evidence based (18%). Additionally, only 19% of the apps incorporated rewarding behavioral changes in their design.
For two of the anxiety plus depression overlap apps, an interventional component was offered but was not accessible to the user in any capacity without payment. These apps are included as part of the total percentage values generated above. App evaluations averaged between 18 and 28 minutes across all app disease states. This average includes the time we allocated to evaluating the apps themselves, evaluating their corresponding websites, and finding and reviewing their privacy policies.
Privacy policy data are summarized in
Table 2. The vast majority of apps stated that they had privacy policies (77%). We had initially planned to quantitatively define the number of privacy policies that stated that they shared data with third parties, sold data to third parties, and so forth. The two authors (A.G. and G.S.) abstracted the data from the individual apps and then created a table with their results. An attorney who is also a CIPP examined the policies and the tables. The attorney discovered that there was significant complexity in how the various app companies described whether and how the data were shared or sold. Most importantly, the attorney noticed that it was difficult for a lay person to understand from a privacy policy, even ones that appeared to be in plain English, how data were shared. For example, some apps stored personal data input (e.g., headache days, depression symptoms), whereas others only shared nonpersonal data, such as the operating system for the device on which the app was used. Additionally, some policies specified that personal user data could never be shared or sold; however, nonpersonal phone data such as app malfunctions could be shared for app improvement functions.
As presented in
Table 3, the app functions varied greatly. The apps most likely to display user-entered data, such as symptoms, emotions, and medications taken, were for migraine (76%), anxiety plus depression (76%), and anxiety (57%). Accordingly, migraine apps were the most likely to generate a data report (72%) followed by anxiety (57%) and anxiety plus depression (48%). These data reports typically compiled previously mentioned user-entered data into a table or graph. Of the apps that enabled users to generate a data report, one anxiety plus depression app required user payment to access this feature. Similarly, three of the migraine apps offered a feature to download or export data logged on the app but required user payment to access it. Many of the apps offered an educational component: depression (100%), Alzheimer’s disease and dementia (63%), and anxiety plus depression (62%). Few apps took advantage of online coaching or social networking. As presented in
Table 2, online coaching was not part of the large majority of the functionality apps (median 0, range 0%−29%). As summarized in
Table 3, only a small minority of apps used social network capability (ranges for the different diseases were 0%−33%).
As previously mentioned, a majority of the apps evaluated offered some kind of interventional component (69%). Stroke and Alzheimer’s apps provided cognitive tasks, such as mental association or memory games. Of the 24% of migraine apps that offered intervention, a majority did so in the form of hypnosis or music. The interventions offered through the depression, anxiety, and anxiety plus depression apps contrast with these methods. Although some apps offered music or hypnosis for relief, these apps were more focused on putting users in control of their symptoms by using techniques like meditation, mindfulness, and deep breathing exercises. Some of these apps incorporated reflection activities for users independently or in conjunction with an artificially intelligent therapist, which correspond to evidence-based cognitive-behavioral therapy (CBT) or progressive muscle relaxation therapy, methods deemed effective for treating such conditions in a clinical context.
Finally, as discussed in the methods, we sought to determine whether the apps were still available and current at the time close to publication of the paper. These results are summarized in
Table 4. As of February 27, 2020, 72 (87%) of the apps were still available on the Google Play and Apple app stores. Of the 72 apps still available for download, 75% were still considered active by our team.
Discussion
In our critical analysis surveying the landscape of the types of apps for the top five neuropsychiatric conditions (stroke, migraine, depression, Alzheimer’s disease and dementia, and anxiety), we observed several key findings. First, there were more apps available for users with migraine, depression, and anxiety than for users with stroke and Alzheimer’s disease and dementia. Second, the apps for stroke and Alzheimer’s disease and dementia generally met few of the recommended features of the APA framework (
18), particularly with respect to functionality and evidence-based components. Lastly, privacy policies could not be easily understood by lay individuals.
It is unsurprising the largest number of apps (and the greatest number of downloads) were for anxiety plus depression and for migraine, given the high disease prevalence in this population as well as the younger age of those affected. Stroke and Alzheimer’s disease and dementia had the fewest apps meeting study criteria. A possible explanation for the low number of apps in these categories is that we excluded apps that were intended for caregiver use; perhaps app developers do not focus on apps for use by participants with these conditions because they believe that they would not be able to use such apps. Also, in the case of stroke, we excluded apps related to motor symptoms, and some apps for stroke were focused more on motor improvements. Research into apps for both Alzheimer’s and stroke may pose their own issues, such as ethical concerns (
21). On the one hand, the low number of apps for Alzheimer’s disease and dementia included in this study is surprising given that one of the WHO’s top 10 research priorities to reduce the global burden of dementia by 2025 is delivery of care and services for people with dementia and their caregivers. Included in their thematic analysis of this category is to “understand the role of assistive and technological devices, including e-health and mobile health technology strategies, for people with dementia and/or their carer(s)” (
22). Research has shown that people with dementia want to be included in research using these technologies (
21). Age itself may not be a barrier, but more research in older adults is needed to determine the best ways to scale and implement these technologies (
23). In particular, we need to consider that everyday technologies often present a barrier for older people, especially those with dementia (
24). Thus, as we consider next steps for the adaptation of these wearables and technologies into everyday life, we need to consider whether there may be additional barriers and whether they can be used under nonoptimal or uncontrolled conditions (
25). An assistive technology taskforce comprised of members of INTERDEM (interdem.org; an interdisciplinary European research network of >160 members, who collaborate to develop and conduct pan-European research on early, timely, and quality psychosocial interventions in dementia) worked together to discuss and reach consensus regarding the current state of technologies for community-dwelling people with dementia. Two of the three areas of global need discussed focused on the persons themselves and were thus relevant to the present study: 1) devices intended to help persons living with dementia to manage their everyday lives across the disease journey, such as electronic calendars and reminders for activities, medication reminders, aids to perform activities of daily life, robots, and navigation systems; and 2) technologies to help people engage in meaningful and pleasurable activities, such as cognitive stimulation and physical activities, as well as technologies to improve social participation, contact, and support. The taskforce members asked, “How can technologies address the heterogeneous needs of persons with dementia? Should technologies be designed specifically for dementia or adapted from mainstream technology? What methods are more efficacious when developing technologies for persons living with dementia?” For more detailed information regarding the consensus recommendations, see Meiland et al. (
21). In summary, though, research into these technologies for people with dementia infrequently dealt with home-based, real-life evaluations. In addition, most technologies were far removed from everyday life experiences and were not mature enough for use under nonoptimal or uncontrolled conditions.
As stated above, we used the APA framework as a guide for the categories we sought to incorporate in our app evaluation model. A majority of the apps in this study required subscription or cost to either purchase the app or access all of its features. Of the eight apps that required initial purchase, seven were from the Apple App Store. This means it is difficult for people using the iOS platform to pick an app without investing in it before being able to fully evaluate if it is suitable to their health needs.
The functionality of many of the apps was limited. With the exception of Alzheimer’s and anxiety plus depression apps, which had the lowest likelihood of being able to share information or achievements with other applications, 57%−75% of apps for all of the other conditions had intercommunicability features. This is surprising given that anxiety plus depression apps were the second most downloaded. Scarcity in intercommunicability features along with the limited capacity to export or download data reports reduces the user’s ability to communicate directly from the app with others—for example, physician or family and friends—and risks fragmenting care by decreasing the feasibility of sharing user-entered data (symptoms, feelings, etc.). Additionally, very few apps provided guidance based on user-entered data, which could be beneficial to users when they are experiencing a mental health crisis or at risk of suicide.
In addition, while the need for evidence-based interventions and apps, as reflected in the APA framework, makes clinical sense, few apps we examined offered such evidence. Migraine apps were the least likely to include any evidence-based intervention, while anxiety and depression apps were the most likely, although even that evidence was often minimal and of a pilot nature. Migraine was also the condition least likely to include any interventional component at all, focusing more on tracking capabilities. The lack of evidence surrounding such interventional components in this study is consistent with findings reported by Byambasuren et al. (
26), who also found limited quality evidence among mHealth apps predominantly geared to obesity, mental health, and diabetes.
The evidence-based intervention most commonly purported to be adapted as an intervention in the app was CBT. Although several apps in our study stated that they used CBT or CBT-based techniques (Pacifica, Youper, Wysa, What’s up?, Depression CBT Self Help, Curable), only the Betterhelp app appeared to deliver therapist-supported Internet-based CBT (ICBT), an established CBT intervention in which an identified therapist provides direct support to a user or patient in need (
27). Although ICBT appears to be an efficacious treatment for anxiety in adults, the evidence comparing therapist-supported ICBT to waiting list, attention, information, or online discussion group only control was low to moderate quality; the evidence comparing therapist-supported ICBT to unguided ICBT was very low quality; and comparisons of therapist-supported ICBT to face-to-face CBT were low quality (
28). Further research is needed to better define and measure any potential harms resulting from these types of treatment.
An emerging intervention in some apps was chatbot technology; four apps used this tool, including Wysa, Youper, and 7 Cups for mental health, and Curable for migraine/general pain. Chatbot technology tries to offer treatment in the form of an ongoing text message conversation. The interest in these bots is due to their often intuitive interface and efforts to establish a therapeutic alliance with the user, compared with often difficult to navigate health apps that do not seek to form a bond with the user. However, one limitation to bots is that they do not always have the correct answers, and few have been clinically evaluated for safety or efficacy.
Additionally, of significant concern was the fact that many of the apps did not have a privacy policy that was easy to understand. The actual privacy rights provided to the user arise from a combination of applicable data laws and regulations, contractual promises made in apps’ terms of services and privacy policies, and the technical mechanics of how an app records and stores information. Our experience in asking lay people to interpret and quantitatively assess how a policy allows or limits the use of personal data showcases the difficulty that a lay person may have in understanding how a privacy policy affects their rights and use of the data.
With regard to the technical mechanics, if the app does not record personally identifiable information (e.g., the app merely plays music and sends no usage data to a data provider), privacy concerns are low. Similarly, if the app records personal information but only stores it on the user’s own device instead of a remote server and never transmits this information elsewhere, privacy concerns are low. However, if personal data, especially personal health information and app usage data, are stored remotely on the app provider’s servers, there is increased risk that the user’s data may be used or sold for purposes not known or desired by the user.
The laws and regulations applicableto any app are specific to the location of the user and the location of the app company and its computer servers. Users may not realize that they have downloaded and used an app that transmits their data to a country with limited data protection laws. If the app company lacks a physical or legal presence in the user’s own country, the user may have little recourse to misuse of their personal data. In this study, many of the apps came from providers outside the United States. The protection granted by local laws varies widely. For example, the California Consumer Privacy Act and the European Union’s General Data Protection Regulation both allow users to demand deletion of their personal data from providers under certain conditions. However, many other jurisdictions do not provide users with the right of deletion, and the only rights available to the user are those contractual rights that the user can enforce locally. Thus, although they are marketed as tools to help alleviate the conditions in question and help users keep track of their symptoms, there needs to be clearer regulation and transparency on how user information is being collected, used, stored, and potentially sold.
Strengths
To our knowledge, this is the first study to examine the landscape of apps targeting the most disabling neuropsychiatric conditions and the patient population for which such apps may be intended. The data obtained were data that a lay person or patient might view when searching for an app to address their health needs. This study also sheds light on the types of interventions offered by such apps, whether the apps themselves are evidence based, and what features are prevalent among apps to facilitate communication between patient and physician.
Limitations
This was a study to better understand the types of apps on the market for various neuropsychiatric conditions. We did not deconstruct any of the apps to better understand the way they work, nor did we do an in-depth critique of the privacy policies. Additionally, beyond the data presented in
Table 4, it is possible that the top 15 apps available on the market for these five specific neuropsychiatric conditions have changed since the initial data collection period from July 30, 2018, to September 2, 2018 (
29); specific app features or evidence supporting interventional components may have changed as well.
Future Directions
Future work may examine whether more apps over time use evidence-based interventions, whether companies make it easier to comprehend privacy policies, and whether there are efforts to promote funding from the government, foundations, and private sector to prioritize mHealth research.