Neuropsychiatric symptoms have also been associated with neuroimaging biomarker abnormalities in the context of brain aging. We, as well as other investigators, have reported associations between neuropsychiatric symptoms and abnormal β-amyloid (Aβ) deposition in persons without cognitive impairment (
10–
14), as well as in individuals with mild cognitive impairment and dementia (
10–
12,
15,
16). A regional progression pattern of amyloid deposition has been reported (
17,
18); however, to date, it is unclear whether amyloid pathology in various brain regions is associated with emotional behavior. A recent study based on a convenience sample of older adults without cognitive impairment may have been the first to report that the association between anxiety symptoms and Aβ burden may vary by the neuroanatomic location of the amyloidosis (i.e., cortical versus subcortical Aβ deposition), with the striatum, thalamus, and amygdala included as subcortical structures in the analysis (
19). Furthermore, given the well-established association between the amygdala and emotion (
20–
22), there may indeed be merit in investigating different subcortical structures, such as the amygdala, striatum, and thalamus, separately.
Discussion
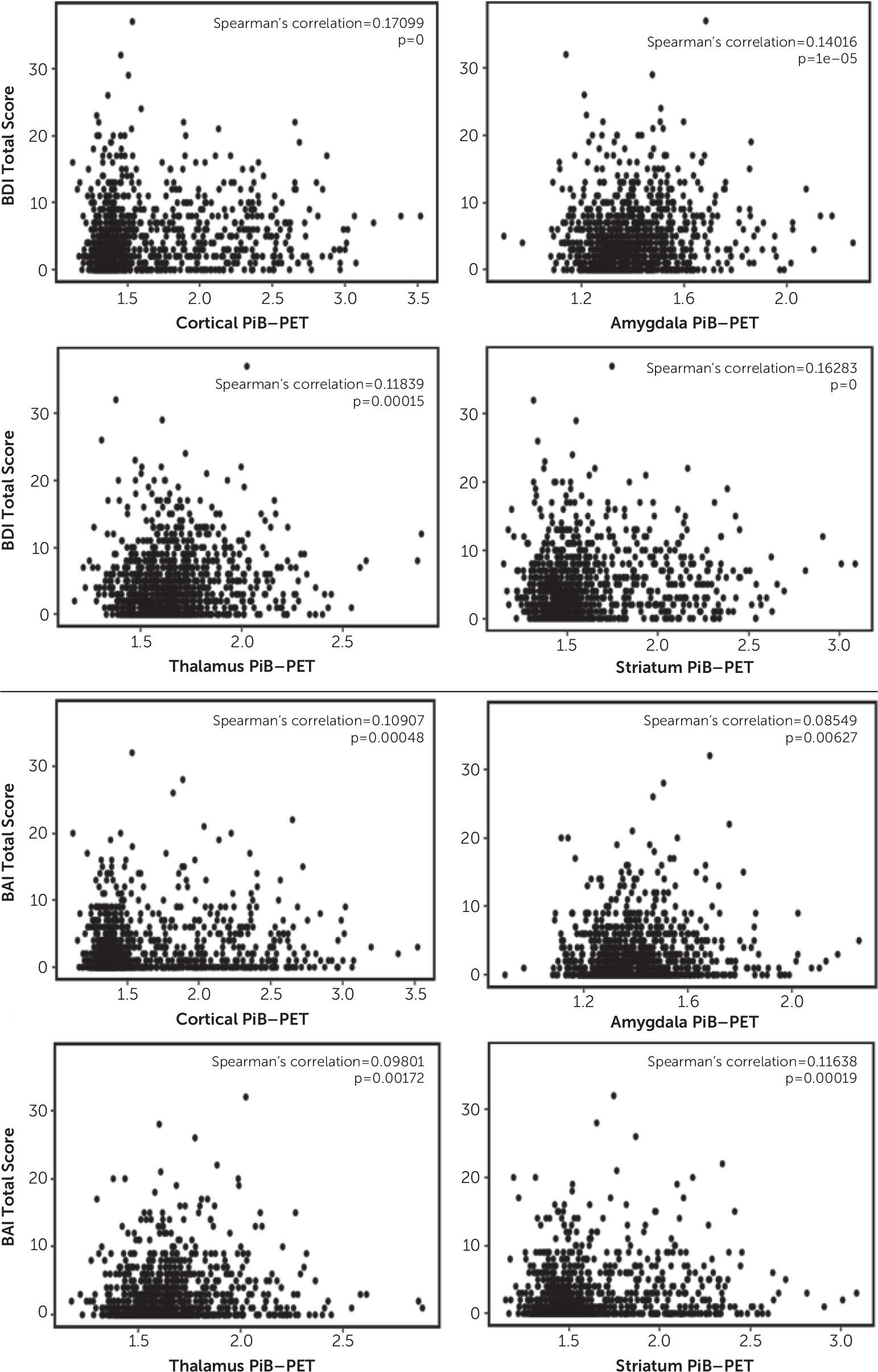
Here, we report a positive association between cortical Aβ deposition, as well as Aβ deposition in the amygdala, striatum, and thalamus, and depressive and anxiety symptoms in community-dwelling older adults without dementia (i.e., higher levels of SUVRs were associated with higher levels of depressive and anxiety symptoms). For example, each 1% increase in the cortical PiB-PET SUVR corresponded with approximately a 0.57% increase in the BDI-II total score and with a 0.34% increase in the BAI total score, on average. In subsample analyses, we observed that PiB-PET SUVRs in the neocortex, amygdala, striatum, and thalamus were associated with depressive symptoms among individuals with mild cognitive impairment. For individuals without cognitive impairment, such an association was observed only in the neocortex and striatum. However, the BAI score was not significantly related to the PiB-PET SUVR in the neocortex or subcortical tissues in either subgroup (without cognitive impairment and with mild cognitive impairment) but was significantly related to the PiB-PET SUVR in the neocortex, striatum, and thalamus in the study group as a whole. The coefficients were rather small, indicating that this association may be more related to the larger sample size and less related to a clinically relevant difference in the amyloid burden.
In a previous observation made by the Harvard Aging Study group, investigators showed that anxiety symptoms were significantly associated with PiB uptake in the striatum, thalamus, and amygdala but not in the neocortex among older adults without cognitive impairment (
19). Thus, their results differ slightly from those of the present study, because we observed associations between both cortical and subcortical Aβ and depressive and anxiety symptoms. Several differences between our study and the study conducted by the Harvard group may account for the discrepancy in findings. For example, the convenience sample in the Harvard study was small and restricted to persons without cognitive impairment, whereas our sample included more than 1,000 community-dwelling nondemented individuals (i.e., without cognitive impairment as well as with mild cognitive impairment). Additionally, the primary outcome of interest in the Harvard study was anxiety as measured by the Hospital Anxiety and Depression Scale and after adjusting for Geriatric Depression Scale (GDS) scores, whereas our outcomes of interest were both depression and anxiety as measured by the BDI-II and BAI, respectively.
In addition, findings from other studies on the association between anxiety or depression and Aβ deposition have been inconsistent. For example, investigators from Sweden reported that anxiety symptoms but not depressive symptoms were associated with cerebral Aβ deposition in a small sample of 104 individuals without cognitive impairment and 53 persons with mild cognitive impairment and that low levels of anxiety were associated with Aβ, which may be a predictor of faster cognitive decline (
11). In line with this, French researchers showed that anxiety symptoms were associated with higher frontal, cingulate, and global cerebral [
18F]-florbetapir uptake in a large sample comprising persons without cognitive impairment and patients with mild cognitive impairment and Alzheimer’s disease; the associations remained significant for frontal and global uptake in the mild cognitive impairment subgroup (
10). In a study using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database, the investigators did not observe an association between the frequency of anxiety or depressive symptoms and Aβ deposition but noted that persons with mild cognitive impairment and amyloid pathology had increased levels of anxiety (
39). Researchers from the University of California Los Angeles reported a positive correlation between GDS scores and lateral temporal Aβ deposition in a sample of individuals without cognitive impairment and with mild cognitive impairment (
12). In another study, investigators used the Australian Imaging, Biomarker and Lifestyle Study of Ageing to examine individuals without cognitive impairment and found that depression was not related to Aβ deposition at baseline, but having high Aβ deposition was associated with a significantly increased risk of new onset of depressive symptoms after 54 months (
40). In a recent study using the construct of mild behavioral impairment, which is characterized by the onset of neuropsychiatric symptoms in older adults, investigators reported that a higher score on the Mild Behavioral Impairment Checklist was associated with global and striatal Aβ deposition in participants without cognitive impairment (
41). Finally, our group reported weak but significant associations between BDI-II and BAI scores and elevated cortical amyloid deposition in community-dwelling participants without cognitive impairment in the Mayo Clinic Study of Aging (
13), as well as higher odds of clinical depression and anxiety among participants with mild cognitive impairment with elevated Aβ deposition (
14). This study builds on our previous research by additionally examining whether there is an association between Aβ deposition in the amygdala, striatum, and thalamus and behavioral symptoms among community-dwelling older adults without dementia.
Few studies have also examined the potential impact of a history of depression. For example, Donovan et al. (
42) reported significantly higher Aβ burden among participants without cognitive impairment with current depression compared with those without a history of depression; they also found a correlation between higher Aβ deposition at baseline and a more pronounced increase in GDS scores. Furthermore, in a study using the ADNI database, investigators found that participants with amnestic mild cognitive impairment and a lifetime history of major depression had increased Aβ deposition in the bilateral frontal lobes compared with those without a lifetime history of depression (
16). Similarly, lifetime history of major depression was associated with higher [
18F]-florbetapir uptake in specific brain regions (
43). In addition to the studies mentioned above, a systematic review of 19 studies found that 15 provided evidence of an association between depression and Aβ deposition in older adults (
44). However, not all studies reported such associations (
15,
45–
47). Conflicting findings may be due in part to differences in methodology, such as smaller sample sizes or different sample compositions (e.g., combinations of participants without cognitive impairment and with mild cognitive impairment versus without cognitive impairment or with mild cognitive impairment only), varying degrees of depression severity between participants from different studies, or different tools used to assess depressive symptoms (e.g., GDS versus NPI-Q).
Results from our study show that, on average, both cortical and subcortical levels of amyloid deposition were associated with depressive and anxiety symptoms in the overall sample and that both cortical and subcortical amyloid deposition were associated with depressive but not anxiety symptoms in participants with mild cognitive impairment. Of note, subcortical Aβ deposition may indicate more advanced amyloidosis beyond the cortex and may be regarded as a marker of pathologic progression (
19,
48,
49). Particularly, according to the five phases of amyloidosis as postulated by Thal et al. (
48), Aβ deposition in neocortical regions reflects phase 1; phase 2 indicates Aβ deposition in allocortical regions, including the limbic system (e.g., the amygdala); and phase 3 indicates Aβ deposition in the striatum and diencephalic nuclei (e.g., the thalamus). As such, subcortical amyloid may merely be a marker of duration of the amyloid deposition process, and there may be no anatomic localization significance of the subcortical abundance of amyloid with regard to its potential association with neuropsychiatric symptoms. Furthermore, it is known that amyloid instigates the degenerative pathways that are in turn responsible for clinical deficits. Therefore, we postulate that amyloid deposition likely does not have a direct effect on depression or anxiety; rather, the degree of Alzheimer’s disease pathology as reflected by amyloid levels (i.e., lesser or greater Thal amyloid phase) may be associated with emotional behavior (
50), even though our cross-sectional results reported here do not support a more pronounced association between Aβ deposition in the amygdala, striatum, or thalamus and behavioral symptoms compared with cortical Aβ deposition. Therefore, more research is needed to investigate the associations between amyloid deposition in different brain regions and neuropsychiatric symptoms.
The strengths of our study include its population-based design, large sample size, and rigorous statistical analysis that adjusted for APOE ε4 genotype status and traditional confounders, including age, which may be important with regard to the effect of age on striatal PiB uptake. In addition, older age and fewer years of education were significantly associated with higher BDI-II and BAI scores, and female sex was associated with higher scores on the BAI but not the BDI-II. APOE ε4 genotype status was not associated with anxiety and depression measures in our sample.
However, there are limitations to our study. As in any cross-sectional study, we were unable to assess causality. Additionally, unlike in clinical samples, the severity of depressive and anxiety symptoms was low in our community-dwelling sample of nondemented older adults. Therefore, we chose to use continuous BDI-II and BAI scores as presumed dependent variables rather than dichotomized outcomes (e.g., BDI-II scores <13 versus scores ≥13), even though we acknowledge the low scores and small variance on the BDI-II and BAI, which may reduce the significance of the association between the PET-PiB SUVRs and the dependent variables in our analysis. In addition, we did not include history of depression or anxiety disorder, as well as potential treatment. Finally, we did not adjust for vascular risk factors, because more research is needed to clarify whether Alzheimer’s disease and vascular pathology are associated (
51) or whether amyloid and vascular pathologies are independent predictors of cognitive decline (
52).