Schizophrenia is a severe and debilitating psychiatric disorder characterized by impairments in thought, affect, attention, and motor coordination, as well as alterations in brain structure and function (
1). There is an increasing focus on brain connectivity and function to determine the causal mechanisms underlying schizophrenia (
2). Resting-state functional MRI (fMRI) is a technique that assesses brain function in the resting state, which is considered a sensitive marker for schizophrenia (
3). Resting-state functional connectivity is an efficient method to examine correlations in activity between different brain regions by measuring blood-oxygen-level-dependent time series. The dysconnectivity hypothesis states that schizophrenia is associated with alterations in neuronal connectivity (
4).
Numerous studies have revealed abnormalities in resting-state functional connectivity between different regions and abnormalities in interhemispheric connectivity among patients with schizophrenia (
5,
6). The left and right hemispheres of the brain are interconnected by the commissural system, of which the corpus callosum is the major white matter tract; split-brain patient studies have demonstrated impairments in various cognitive functions (
7). Moreover, a behavioral study showed that healthy participants exhibited superior information-processing performance when the task was presented bilaterally rather than unilaterally (
8). However, in some tasks dependent on both hemispheres, patients with schizophrenia do not display the bilateral advantage observed with healthy participants (
9). Furthermore, several studies have shown that schizophrenia is associated with abnormal functional lateralization in frontal, temporal, parietal, and occipital areas (
10,
11). Mohr et al. reported abnormalities in interhemispheric interactions among individuals with schizophrenia by using event-related potentials (
12). There is also extensive research with diffusion tensor imaging showing abnormalities of the corpus callosum in schizophrenia, with most investigators reporting reductions in fractional anisotropy in the corpus callosum (
13). In a more recent diffusion tensor imaging study, investigators found interhemispheric dysconnectivity among not only patients with schizophrenia but also their relatives (
14). Taken together, these findings indicate that schizophrenia involves aberrant interhemispheric cooperation due to abnormalities in interhemispheric structural connectivity.
Auditory verbal hallucinations (AVHs) are a typical symptom of schizophrenia, with approximately 60%−80% of patients experiencing AVHs (
15). Among most of these patients, AVHs respond to antipsychotic pharmacotherapy; however, in a substantial proportion of patients, AVHs are treatment resistant and continue to be experienced. Studying the mechanisms underlying AVHs will facilitate understanding of the etiopathogenesis of auditory hallucinations and provide a foundation for the treatment of schizophrenia and AVHs. A popular theory suggests that AVHs are caused by abnormalities in brain regions associated with language, such as hyperarousal of the auditory cortex (
16). Studies have suggested that among patients with schizophrenia, the boundary between inner speech and external sounds is blurred (
17). Furthermore, an increasing number of studies are indicating that schizophrenia may be related to alterations in the development of left-brain dominance for language (
18). It is well established that language functions are highly asymmetric, and therefore interhemispheric interactions likely play an important role in the pathophysiology of schizophrenia with AVHs. According to the interhemispheric miscommunication theory of AVHs, altered microstructure of the interhemispheric auditory pathways and disrupted functional gamma-band synchrony between the two auditory cortices play an important role in AVHs (
19).
If AVHs are caused by abnormalities associated with language function, interhemispheric connectivity and measures of VMHC that represent the resting-state functional connectivity between homotopic voxels in the two hemispheres will differ between patients with schizophrenia and AVHs and those without AVHs. In the present study, we compared interhemispheric functional connectivity among patients with schizophrenia and refractory auditory verbal hallucinations (RAVH group), patients with schizophrenia without AVHs (non-AVH group), and healthy control participants (HC group). Considering previous evidence, we hypothesized that more aberrant interhemispheric connectivity would be observed among patients with schizophrenia than among healthy control participants. Moreover, we hypothesized that the patients with schizophrenia and RAVHs and those without AVHs would exhibit distinct functional connectivity deficits because of differences in language-related symptoms. Finally, we examined whether VMHC measures were associated with clinical characteristics among patients with schizophrenia.
Methods
Participants
This study was reviewed and approved by the local institutional review board, and we followed the principles established by the Declaration of Helsinki. Data were collected between June 2017 and May 2021. A total of 57 inpatients with a DSM-IV diagnosis of schizophrenia were recruited from the local psychiatric hospital. All participants satisfied the following inclusion criteria: diagnosis of schizophrenia on the basis of the Structured Clinical Interview for DSM-IV criteria; stable administration of any psychotropic medications for at least 8 weeks before the start of the study; presence of clinical symptoms without remission despite administration of an adequate dosage and course of antipsychotic medication (
22); right-handedness; and native Chinese speaker. The exclusion criteria were as follows: history of significant head trauma or neurological disorder; alcohol or drug abuse; focal brain lesions on T1- or T2-weighted fluid-attenuated inversion-recovery magnetic resonance images; recent aggression or other forms of behavioral dysfunction; head motion during the resting-state fMRI scan that exceeded 3 mm in translation or 3° in rotation; and diagnosis of another psychiatric disorder before the diagnosis of schizophrenia or before the scan.
The patients with schizophrenia were classified into two groups: the RAVH group comprised patients who reported AVHs at least five times a day for the past 8 weeks (N=34), and the non-AVH group comprised patients who had never experienced AVHs or had not experienced AVHs during the 5 years before recruitment (N=23). The one patient in the non-AVH group who had experienced AVHs more than 5 years before the present study was excluded from this study because of excessive head motion; therefore, no patient in the non-AVH group had actually experienced AVHs. All patients were assessed with the Positive and Negative Syndrome Scale (PANSS) (
23). Patients in the RAVH group also completed the Auditory Hallucination Rating Scale (AHRS). Two senior clinical psychiatrists performed these ratings; the interrater reliability between these two psychiatrists was greater than 90%. All patients in the RAVH group had refractory AVHs, which were defined as persistent daily hallucinations without remission despite the administration of antipsychotic medication at an adequate dose for at least 12 weeks. All patients in the RAVH group experienced only verbal hallucinations.
Twenty-eight healthy volunteer participants with no history of neurological or psychiatric illness were randomly recruited from the local community. Individuals in this group exhibited no gross abnormalities on brain MRI.
MRI Data Acquisition
Structural and functional MRI with the three study groups was performed with a 1.5-T MRI scanner (General Electric Medical System, Boston) and a 16-channel head coil. During the resting-state fMRI scans, participants were instructed to lie still in the scanner, keep their eyes closed, relax, think of nothing in particular, and not fall asleep. During the scan, participants were monitored for wakefulness by specialized staff; after the scan, participants were asked whether they had remained awake throughout the scan. We used foam padding to minimize head motion and earplugs to dampen scanner noise. Resting-state fMRI time series were acquired with an echo-planar imaging sequence with the following parameters: repetition time=2,000 ms; echo time=40 ms; flip angle=85°; voxel size=4.0 mm × 4.0 mm × 4.0 mm; field of view=256 mm × 256 mm; 28 slices across the whole brain; and 216 volumes. A high-resolution three-dimensional T1-weighted image was acquired by using a gradient-echo sequence with the following parameters: repetition time=9.5 ms; echo time=3.1 ms; flip angle=20°; voxel size=1.0 mm × 1.0 mm × 1.0 mm; slice thickness=1.1 mm, field of view=256 mm × 256 mm; and number of slices=176.
Image Preprocessing
Resting-state fMRI data were preprocessed with the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox, version 2.3 (
http://rfmri.org/DPARSF), and the Statistical Parametric Mapping software package (
http://www.fil.ion.ucl.ac.uk/spm). The first 10 volumes were removed to eliminate the nonequilibrium effects of magnetization and to allow participants to adapt to the scanner environment. Data were then corrected for the time delay and head motion. The motion thresholds were set at translation of 3.0 mm and a rotation of 3° in any direction. Data for two of the patients in the non-AVH group were excluded from further analysis because of excessive head movement. After motion correction, anatomical images for each participant were registered to his or her realigned echo-planar images. After realignment, the T1 image of each participant was normalized to a structural brain template, and the functional images were registered to the corresponding T1 images; images were then normalized to the standard space and resampled to 3 × 3 × 3 mm
3. We then used a 6-mm full width at half maximum Gaussian kernel for spatial smoothing. We performed linear trend removal and applied a temporal bandpass filter (0.01–0.08 Hz). Linear regression was used to control for confounding factors, which included six head motion parameters (three translation parameters and three rotation parameters) and ventricle and white matter signals.
VMHC Analysis
VMHC analysis was conducted by using the Resting-State fMRI Data Analysis Toolkit (REST;
http://resting-fmri.sourceforge.net). We calculated VMHC following protocols described previously by Zuo et al. (
20). First, the mean normalized T1 image was created by averaging all normalized T1 images. This image was then averaged with its mirrored left-right version to produce a group-specific symmetrical template. Second, the normalized T1 images of each participant were registered to the symmetric template, and the registration parameter was applied to each participant’s preprocessed functional data. Finally, Pearson’s correlation coefficient between the time series of each pair of mirrored interhemispheric voxels was used to calculate each participant’s homotopic connectivity coefficient. The Fisher Z-transformed correlation values were then referred to as the VMHC.
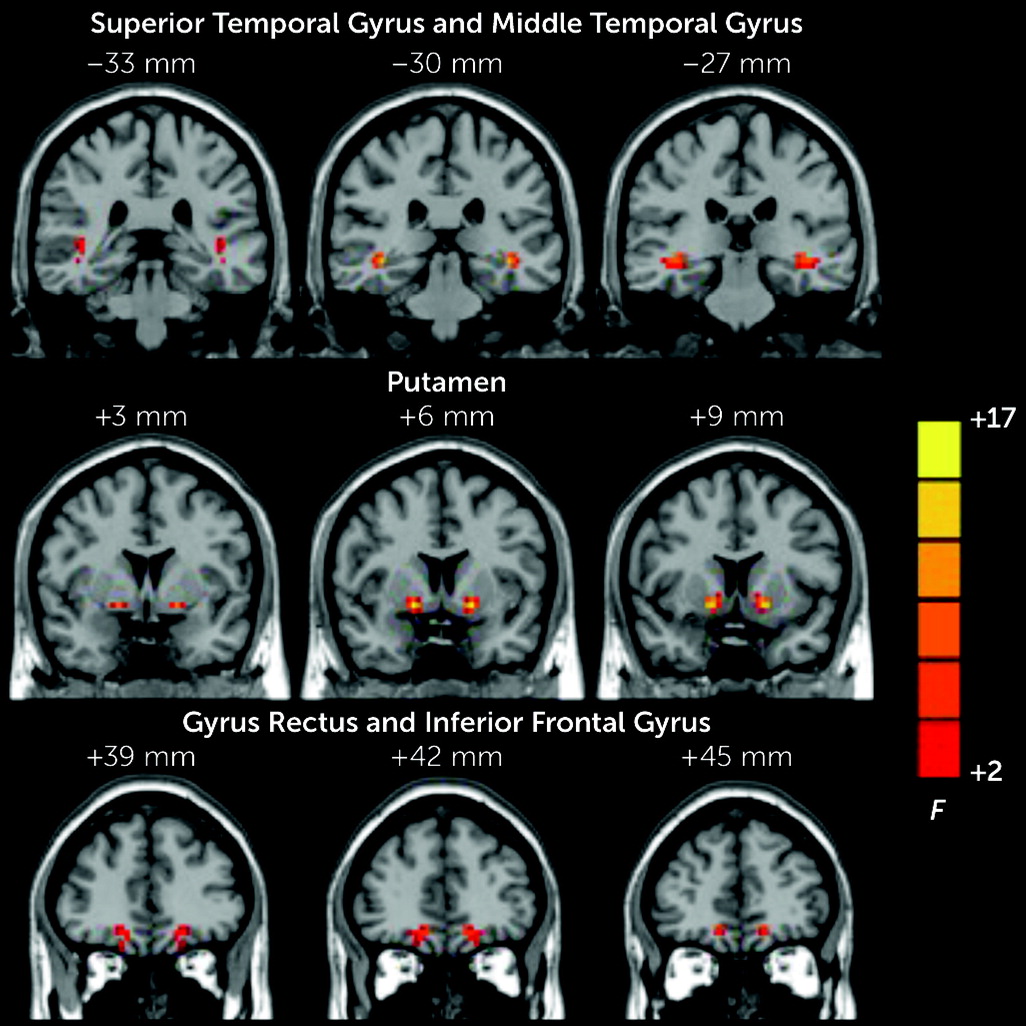
We entered the transformed Z-maps of each participant into a random-effects one-way analysis of variance (ANOVA) to identify differences in VMHC among the three study groups, and age, gender, and mean relative displacement of the head were used as covariates. By using the significant brain regions from the ANOVA as a mask, we performed post hoc comparisons between each of the three groups with two-sample t tests. The statistical threshold was set at a corrected p value <0.05 (determined by Monte Carlo simulations, uncorrected p<0.005, with a cluster size >25 voxels). In addition, mean VMHC signals were extracted from a 6-mm sphere within each significant region revealed by the one-way ANOVA. Peak coordinates of the cluster were set as the center of the sphere.
Statistical Analyses
All analyses of demographic data were performed with SPSS software. A chi-square test was used to compare gender distribution between groups, and two-sample t tests were used to compare illness duration, medication, total PANSS score, PANSS negative subscale scores, and PANSS positive subscale scores between the two patient groups. One-way ANOVAs were used to compare age and education among the three study groups. A two-tailed p value <0.05 was considered statistically significant.
We used REST software to conduct the correlation analyses. Pearson’s correlations were used to analyze associations between the mean VMHC of the significant brain regions and clinical variables, such as total PANSS score, PANSS negative subscale scores, and PANSS positive subscale scores, among all patients with schizophrenia. We also examined the relationship between AHRS scores and the mean VMHC of significant brain regions among the patients with schizophrenia and AVHs. The statistical threshold was set at a p value <0.05 after false discovery rate correction.
Discussion
In this study, we explored the correlations between homologous brain regions among patients with schizophrenia and RAVHs, patients with schizophrenia and no AVHs, and healthy control participants. We found that the patients with schizophrenia and RAVHs had aberrant interhemispheric connectivity in several brain regions, which included the gyrus rectus, IFG, STG, MTG, and putamen. These findings are consistent with those of previous studies that reported abnormalities in interhemispheric interactions in schizophrenia by using a variety of methods, such as behavioral, electrophysiological, and task-based fMRI investigations (
12). Our study extended previous research by exploring abnormalities in interhemispheric interactions among patients with schizophrenia with and without AVHs.
We used rigorous criteria to select the patients with schizophrenia: all patients in the RAVH group had refractory AVHs, had no other types of hallucinations except verbal hallucinations, and had never been diagnosed with another psychiatric disorder, whereas all patients in the non-AVH group had never experienced AVHs. Moreover, all participants were native Chinese speakers, and they had to be right-handed in order to minimize differences in interhemispheric interactions that depend on the strength and consistency of handedness (
25).
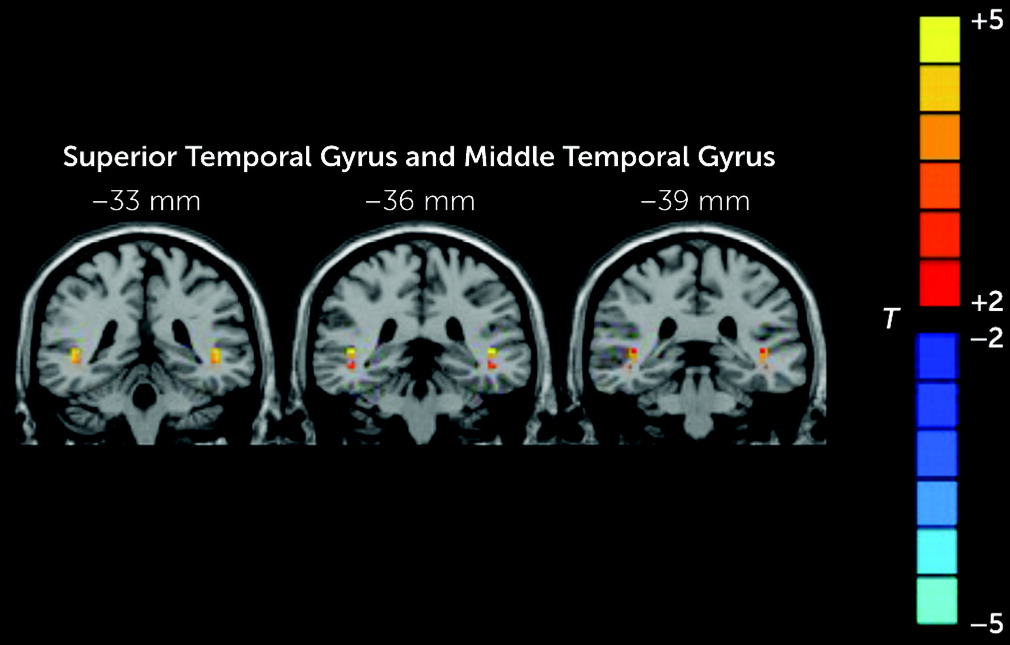
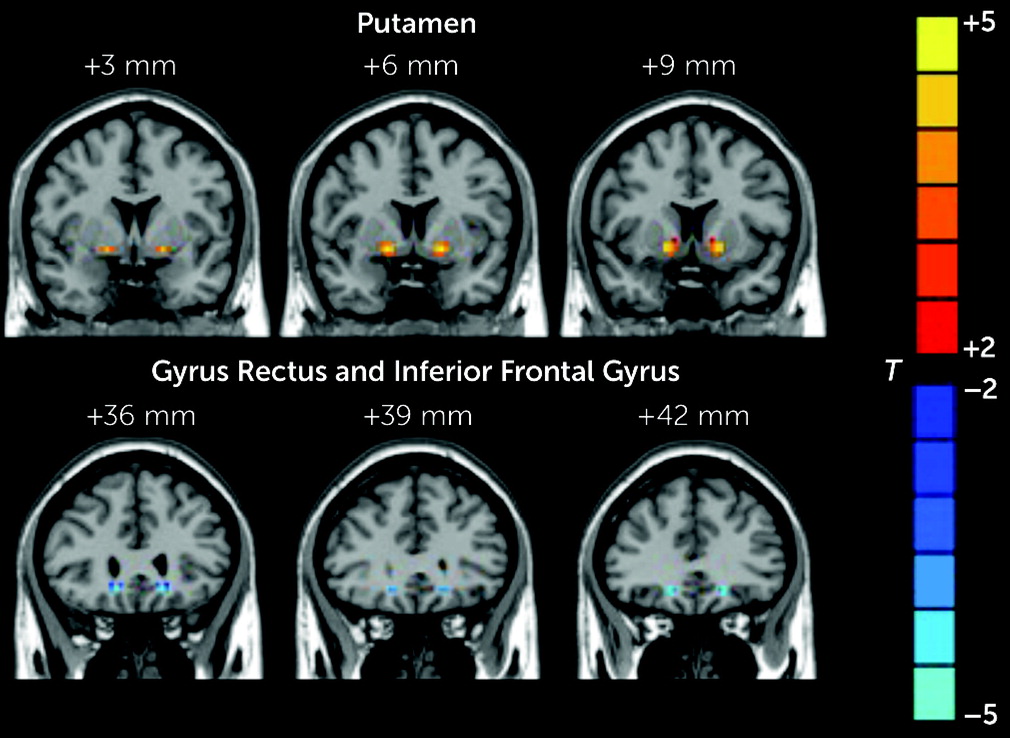
The patients in the RAVH group showed regions of lower and higher VMHC compared with the other two study groups. Specifically, compared with the healthy control group, the RAVH group showed significantly lower functional connectivity between the left and right IFG and between the left gyrus rectus and its right homolog. However, no differences were observed between the RAVH and non-AVH groups. The IFG is one of the frequently studied brain regions in research investigating the mechanisms underlying auditory hallucinations among patients with schizophrenia (
26). Hoffman et al. found that functional connectivity seeded from Wernicke’s area to the left IFG was significantly greater among patients with AVHs compared with that among patients without AVHs but not compared with that among healthy control participants (
27). In a similar study, Chang et al. found that the AVH group had significantly higher VMHC values between the left and right IFG than the control group, and the non-AVH group had intermediate VMHC values (
28). This is inconsistent with the findings of the present study, which may be attributed to two features of the studies. First, the selection of participants differed between studies. Chang et al. included patients with AVHs who had experienced AVHs at least once per day for the past 4 weeks and patients without AVHs who had never experienced AVHs or had not experienced AVHs during the 2 years before study recruitment. In contrast, we included patients with RAVHs, and the inclusion criteria for the non-AVH group required a longer duration of not experiencing AVHs. Second, the measurement of VMHC in our study was focused on functional connectivity between homotopic regions, whereas the previous study, which did not focus on the relationship of homotopic regions, explored abnormal functional connectivity of the left IFG with other regions. Our analysis did not examine whether functional connectivity between the IFG and all regions except the right IFG was normal, and dysconnectivity was not defined by an increase or decrease but rather as aberrant connectivity (
4).
The orbitofrontal cortex can be divided into five subregions: the gyrus rectus and the medial, anterior, posterior, and lateral orbital gyri (
29). A cross-sectional study showed that patients with first-episode schizophrenia had pronounced gray matter volume decline in the gyrus rectus (
30). In a recent study of neurotypical adults, the investigators found that asymmetry of the gyrus rectus may predict trait alexithymia, cognitive empathy, and social function (
31). Furthermore, Faria et al. used a new method to explore the relationship between white matter architecture and cognition among patients with first-episode schizophrenia and found that executive function correlated with fractional anisotropy in the white matter adjacent to the gyrus rectus (
32). Previous studies of the gyrus rectus have primarily focused on the association between brain structure and executive function; thus, we speculate that dysconnectivity of the bilateral gyrus rectus disrupts the integration of executive functions between the left and right hemispheres, which exacerbates internal language disorders.
The VMHC values of the MTG and STG were lower in the RAVH group than in the non-AVH group. Onitsuka et al. reported that patients with schizophrenia and AVHs have lower gray matter volume in the left STG and MTG than those without AVHs, and these lower volumes correlated with a greater severity of hallucinations (
33). The MTG is an important component of the language pathway and is connected to the frontal lobe and other brain regions responsible for language (
34); therefore, it is thought to be involved in the development and experience of AVHs. According to the feed-forward neurocognitive model, the MTG is involved in speech processing when the perceived results of inner speech activity do not match the predicted results (
35).
The temporal lobe, especially the left STG and MTG, plays a key role in the brain’s language network (
36). Okuneye et al. found that among patients with bipolar disorder, increased interhemispheric connectivity between higher-order temporal-parietal auditory/language regions was related to a greater severity of hallucinations (
37). Guo et al. reported lower VMHC values in several brain regions, such as the STG and precuneus, among drug-naive patients with first-episode paranoid schizophrenia; furthermore, VMHC values of the STG were negatively correlated with PANSS positive and negative subscale scores and the total score (
5), which is inconsistent with the findings in our study. Such discrepancies may be due to differences in clinical characteristics of the populations selected, such as symptom severity and illness duration. Reduced volumes of the STG and MTG indicate that the neurodevelopment of the language network and associational cortex contributes to the pathophysiology of schizophrenia (
38).
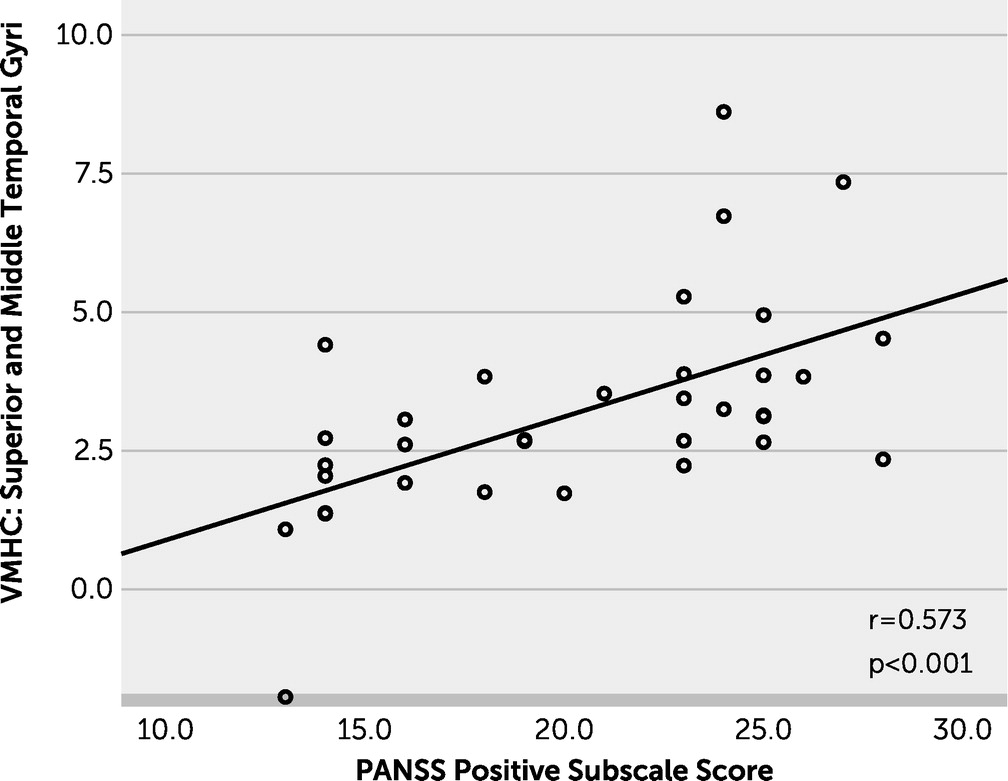
The abnormal VMHC values of the STG and MTG may be due to reduced volumes of these regions. Notably, in our study, it was only the RAVH group, not the non-AVH group, that showed significantly lower VMHC values in the MTG when compared with the healthy control group. In addition, significant positive correlations were observed between VMHC values of the STG and MTG and PANSS positive subscale scores, which indicate that the reduced VMHC in the STG and MTG may partly contribute to abnormalities in language function and psychiatric symptomatology.
The VMHC values of the putamen were higher in the RAVH group than in the non-AVH group. Speech production increases activation in the left putamen, and the left putamen is more related to the initiation and execution of overt speech relative to covert speech (
39). The putamen is part of the basal ganglia, and the cortico–basal ganglia–thalamo cortical circuit plays an important role in various functions, such as motor, executive, and motivation functions (
40). van Tol et al. found lower left putamen gray matter volume among patients with schizophrenia and AVHs than among those patients without AVHs (
41). In our study, we found higher functional connectivity between the left putamen and its right homolog, which we speculate was due to a disruption in the laterality specificity of the function of the left putamen, resulting in higher homotopic connectivity of the bilateral putamen. Dysconnectivity hypotheses suggest that patients with schizophrenia have abnormal functional integration of neural systems (i.e., dysconnectivity). The term dysconnectivity indicates an abnormal (rather than decreased) functional integration among brain regions, and our findings support such a theory. Although our method of VMHC limits the number of comparisons, the findings align with interhemispheric miscommunication theory.
Finally, we did not identify any regions with VMHC values that were significantly correlated with AHRS scores. We suggest several reasons for this finding. First, our sample size was not large enough to have sufficient power; the field of the 1.5-T scanner is not strong enough to detect more precise relationships. Second, use of only the AHRS was not enough to sufficiently reflect the experience of AVHs among patients with schizophrenia due to the complexity of these hallucinations in schizophrenia. Therefore, future studies should combine additional instruments, such as the Psychotic Symptom Rating Scales, to assess the symptoms of AVHs. A previous study, which also used the VMHC method, showed that no significant correlations with AHRS scores were found among patients with AVHs; the difference is that the patients in that study were antipsychotic naive and had first-episode schizophrenia (
28). This suggests that there may be a more complex and deeper relationship between VMHC values and symptoms of AVHs among patients with schizophrenia, and the relationship may be obscured by the nature of the symptoms.
There are several limitations to the present study. First, the left and right hemispheres of the human brain are not symmetrical. We applied a symmetrical standard template and smoothed our imaging data to improve correlations between regions in the two hemispheres. In future research, this limitation can be resolved by developing more advanced algorithms to define functional homotopy (
42). Second, a longitudinal study is necessary to examine whether these alterations in neural activity are associated with normal neurobiological processes in schizophrenia or whether they are specifically associated with hallucinations. Third, we used a 1.5-T scanner, and perhaps the field was not strong enough, resulting in lower signal-to-noise ratios, and it may be possible to achieve a more widespread result at a higher field. Additionally, in some areas, such as the orbitofrontal cortex, data obtained with this scanner is vulnerable to motion and sinus air artifacts that can corrupt fMRI signals. However, in the preprocessing pipeline of resting-state fMRI analysis, we did not include the field map distortion correction step, which could improve the detection of functional connectivity (
43). Therefore, we should complete these steps to improve signal quality in future studies. In addition, our rigorous criteria for selecting patients with RAVHs limited our sample size, which may have lowered the statistical power. In the future, we plan to study larger samples to enable the detection of more novel and subtle differences. Moreover, because of the rigorous criteria, all patients were taking psychotropic medication, which may have increased variability. However, we ensured that patients were taking stable doses of psychotropic medication for at least 8 weeks. Finally, we only selected patients with RAVHs to minimize heterogeneity; however, this may have limited the generalizability of our results to other clinical subtypes of schizophrenia.