Given the evolving understanding of PASC, clinicians are faced with the challenge of having to assess and manage patients with neuropsychiatric symptoms linked to COVID-19.
Evaluation
Initial evaluations should be comprehensive and cover all possible biological and psychosocial factors. COVID-19 acute respiratory and neuropsychiatric symptoms, and their presumed pathophysiological mechanisms (i.e., embolic stroke, hypoxic-ischemic brain injury) and treatments (i.e., steroids, other antimicrobial therapies), help clarify the extent and severity of the initial insult. Time correlation to a positive COVID-19 polymerase chain reaction antigen (or antibody) test helps solidify etiological correlation to acute symptoms; however, variable access to testing needs to be considered, alongside the usual sensitivity and specificity of the particular test, combined with clinical compatibility of the symptoms with COVID-19. As in any comprehensive assessment, a detailed medical and neuropsychiatric background history can uncover preexisting conditions that may be predisposing, although the significance of preexisting conditions for the development of PASC symptoms is not yet fully understood. Medical, neurological, and psychiatric conditions that could alternatively cause similar symptoms need to be considered. This is relevant because many neuropsychiatric PASC symptoms can be explained by other etiologies, and the onset after a COVID-19 infection may be coincidental, as is the case with other conditions (
62).
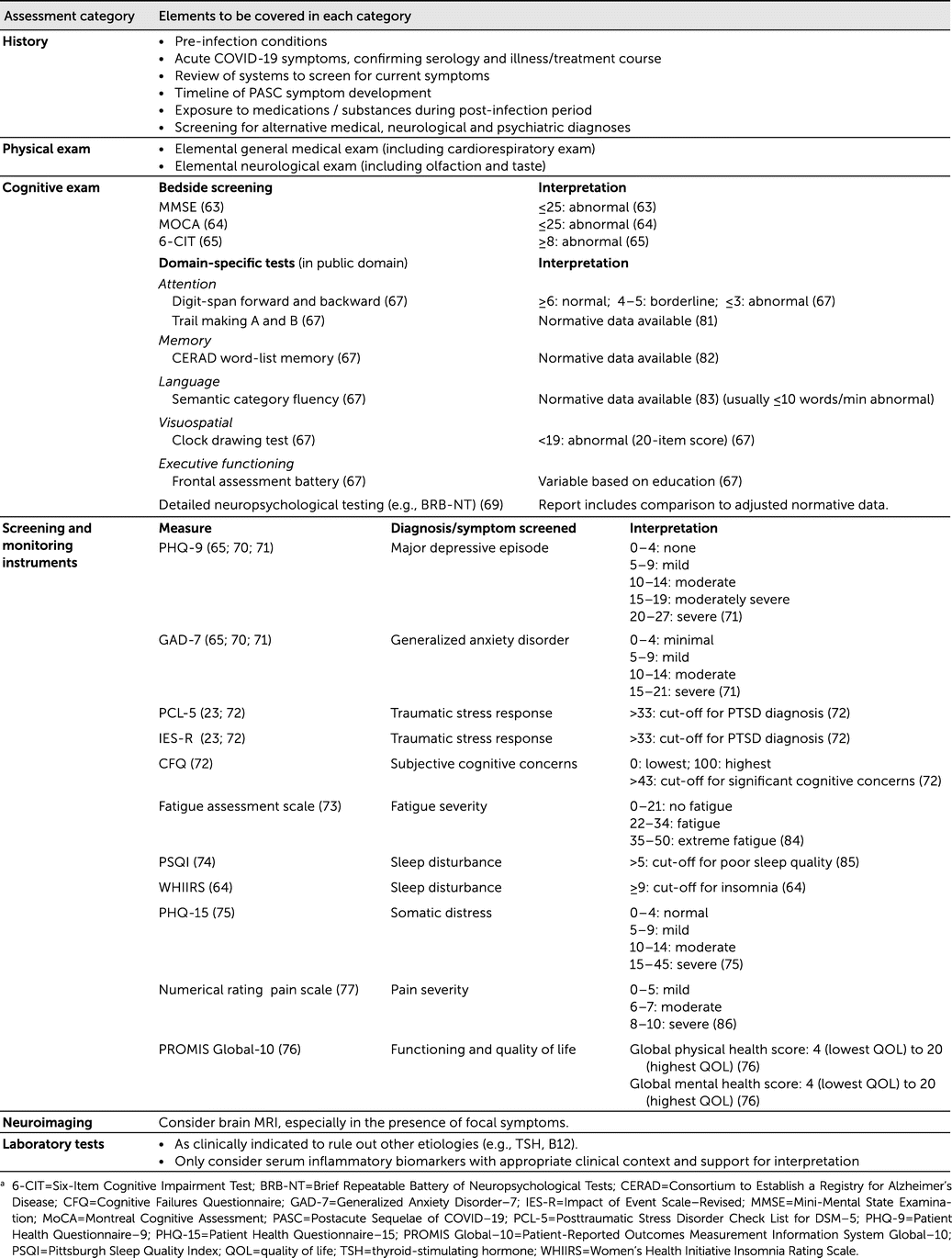
A thorough examination, including both broad objective, as well as subjective, cognitive assessment helps characterize symptoms. Cognitive screening measures, such as the MMSE, MoCA and the six-item Cognitive Impairment Test, or the domain-specific Frontal Assessment Battery, have been used in post-COVID or PASC patient samples to detect cognitive impairment (
63–
66). When evidence for the use of a cognitive test in this clinical population is not yet available, other cognitive measures may be considered and should be consistent with recommendations from the American Academy of Neurology Behavioral Neurology Section Workgroup (
67) or the DSM-5 (
68). Examples of domain-specific cognitive tests in the public domain that align with those recommendations are offered in
Table 1. Further detailed neuropsychological assessment may help quantify cognitive deficits and is useful for longitudinal comparison of performance. The Brief Repeatable Battery of Neuropsychological Tests consists of a group of tests that evaluate verbal memory (Selective Reminding Test), visuospatial memory (Spatial Recall Test), attention and processing speed (Symbol Digit Modalities Test), working memory and sustained attention (Paced Auditory Serial Addition Test), and semantic verbal fluency (Word List Generation Test). This battery has been used in post-COVID patients (
69).
Self-rating symptom- or syndrome-specific screening instruments are ideal to highlight disabling symptoms and to monitor progress over time. Many such instruments have already been used in patients after the acute phase of a COVID-19 infection. Examples include the Patient Health Questionnaire-9 (PHQ-9) for major depressive episode symptoms (
65,
70,
71), Generalized Anxiety Disorder-7 (GAD-7) for GAD (
65,
70,
71), Posttraumatic Stress Disorder Check List for DSM-5 or Impact of Event Scale–Revised (IES-R) for traumatic stress response (
23,
71,
72), Cognitive Failures Questionnaire for subjective cognitive complaints (
72) (to be supplemented by objective cognitive evaluation), Fatigue Assessment Scale for fatigue severity (
73), Pittsburgh Sleep Quality Index or Women’s Health Initiative Insomnia Rating Scale for sleep impairment (
64,
74), and the Patient Health Questionnaire-15 (PHQ-15) for somatic distress (
75). The Patient-Reported Outcomes Measurement Information System Global-10 (PROMIS Global-10) measures impact on functioning and quality of life and provides scores on global physical and mental health (
76). Formal assessment of pain severity with a numerical rating scale (
77), included in the PROMIS Global-10, can be useful to guide treatment of pain syndromes, including headache.
Most studies of patients with PASC rely on subjective report of neurological symptoms, such as dysosmia, dysgeusia, or sensorimotor dysfunction (e.g., paresthesia, numbness, and weakness) (
8). In addition to screening for such symptoms, an elemental neurological exam will help corroborate reported cranial nerve abnormalities (
78), identify focal deficits and neuropathies (
79), and/or provide positive signs of functional neurological disorder (
80).
As of now, there is no pathognomonic neuroimaging sign typical of neuropsychiatric PASC. However, clinicians should have a low threshold to consider neuroimaging during an initial assessment, especially in the presence of focal symptoms (whether motor, sensory, or cognitive). As of now, the clinical significance of abnormal laboratory findings for patients with PASC symptoms remains unknown (other than screening for alternative etiologies). Laboratory exams screening for inflammatory biomarkers (i.e., serum cytokines such as c-reactive protein, IL-1 beta, IL-6, IL-10, TNF-alpha) may eventually provide a link to a possible neuropathological mechanism to explain some PASC symptoms but are not currently justified clinically outside of a research context.
Table 1 lists clinical information, cognitive tests, and screening scales informed by existing evidence that can assist during the initial evaluation of patients with PASC (
81–
86).
Treatment
Until a clear pathological mechanism for PASC symptoms is identified, the therapeutic approach remains etiologically agnostic and symptom focused. If a secondary etiology is known to play a role in the development of symptoms (e.g., embolic stroke, steroid-induced symptoms, functional etiology that is based on positive signs), then clinicians should treat those symptoms as indicated by the identified etiology. Other neuroinvasive viral infections with long-term neuropsychiatric sequelae offer limited therapeutic guidance because evidence for treatment is lacking (
16), and even if such evidence existed, it is unclear whether pathogenic mechanisms would be similar enough across viruses for therapeutic benefit to translate to PASC.
Rehabilitative and behavioral (e.g., mindfulness-based) interdisciplinary approaches can be useful to reduce fatigue and pain symptoms, as shown by transdiagnostic evidence of efficacy in other neurological diagnoses (
87,
88). A gradual pacing of activities to develop cognitive and physical tolerance can alleviate symptom severity and impact on functioning among post-COVID patients (
6). Cognitive rehabilitation can help manage deficits in attention, executive functioning, memory, emotional regulation, and self-efficacy, as seen in evidence from other neuropsychiatric conditions (
89).
Management of mood, anxiety, and trauma-related symptoms should follow evidence-based recommendations per existing clinical practice guidelines (
90,
91). There is no current evidence to suggest that treatment of emotional disturbances in the context of PASC should differ substantially from the management of their equivalent primary psychiatric diagnoses. This, however, may change as we better understand the underlying pathophysiology of PASC and its therapeutic implications.
There is also limited evidence to support the use of psychopharmacological agents to treat other neuropsychiatric PASC symptoms, even by analogy to diagnoses with overlapping symptoms. Medications that promote wakefulness through histaminergic neurotransmission, such as modafinil or armodafinil, have inconclusive evidence to treat fatigue across neuropsychiatric conditions (
92). For cognitive symptoms, the benefit of medications that increase dopaminergic neurotransmission (e.g., methylphenidate) in other neuropsychiatric conditions, such as traumatic brain injury, is limited to short-lived improvements in attention (
93). Clinicians may therefore need to engage in sequential empirical trials of medications to address specific PASC symptoms, such as fatigue or cognitive complaints, especially if nonpharmacological interventions are unsuccessful. Rational psychopharmacotherapy principles should be practiced. For instance, dual-purpose therapies (e.g., amitriptyline for any combination of insomnia, headache, pain, depression, or anxiety) can minimize exposure to multiple drugs.
We recommend measuring symptom severity over time to assess therapeutic response. Depending on impact on vocational and social functioning, interventions should aim to reestablish preinfection functioning when possible.
Table 2 outlines suggestions for the management of patients with neuropsychiatric PASC symptoms, following evidence-guided information when possible.