Out-of-hospital cardiac arrest (CA) is estimated to occur in 320,000–334,000 adults per year in the United States.
11 At least some degree of cognitive impairment is commonly seen in survivors of CA.
6,12–15 Pathophysiologic mechanisms leading to cognitive impairment and neurologic dysfunction remain unclear, because this is a complex, multistage injury.
16–18 Cerebral blood flow plummets immediately after CA, causing a global hypoxic-ischemic state. Multiple mechanisms that may contribute to injury at this stage have been identified. In addition to substrate depletion, excitotoxicity, acidosis, and increased levels of injurious substances (e.g., cytokines and reactive oxygen species) occur. Successful cardiopulmonary resuscitation and restoration of circulation improve oxygen and energy availability, but they can also trigger reperfusion-related injury mechanisms (e.g., formation of microthrombi, systemic inflammatory state, or secondary injury cascades). Therapeutic hypothermia may be used as a neuroprotective intervention in patients who remain comatose after the return of spontaneous circulation.
16–18 In addition, a critical illness state may develop, compounding injury.
13 A recent commentary emphasized the potential of broad implementation of high-quality, integrated, multidisciplinary care to optimizing both survival and longer-term outcomes after CA.
19Predictors of Survival and Gross Neurological Outcome
Some factors occurring during or acutely after CA are shown to accurately predict early outcome. A meta-analysis of 79 studies (1950–2008) reported an overall survival rate to hospital admission of 23.8% and survival rate to hospital discharge of 7.6%.
20 More recent studies indicate that survival time is improving at many centers, with overall rates of survival to hospital discharge of 10.4%−15.5%.
21–23 In the meta-analysis, survival to hospital discharge was more likely if the arrest was witnessed either by emergency medical services or a bystander, if the patient received bystander cardiopulmonary resuscitation, or if the initial cardiac rhythm was ventricular fibrillation or ventricular tachycardia (shockable rhythms).
20 In recent studies, rates of survival to hospital discharge for bystander-witnessed CA with a shockable rhythm ranged from 28.9% to 34.9%.
11,21,22 Data vary regarding age as a predictive factor for outcome after CA. One systematic literature review found that the survival rate to hospital discharge in elderly patients (adults aged >70 years) was only 4.1%.
24 However, a recent study in elderly patients (aged >75 years) found that neurologic outcome depended on CA characteristics (e.g., time from collapse to resuscitation ≤3 minutes), rather than age.
25Therapeutic hypothermia is recommended for adults who are resuscitated after CA caused by ventricular conduction abnormalities, and it may be considered for certain patients with pulseless electrical activity or asystole.
26 Since the introduction of therapeutic hypothermia for these types of CA, successfully resuscitated patients have had improved neurologic recovery. An early review of the research noted that therapeutic hypothermia (32°C–34°C) was shown to improve both short-term survival and gross neurologic outcomes of unconscious patients after CA.
13 The recent large international randomized Target Temperature Management Trial comparing therapeutic hypothermia of 33°C with 36°C found no significant difference in all-cause mortality or neurologic outcomes at 6 months.
27 The authors noted that other studies have also found benefit from milder cooling and that prevention of fever might be one important mechanism of action.
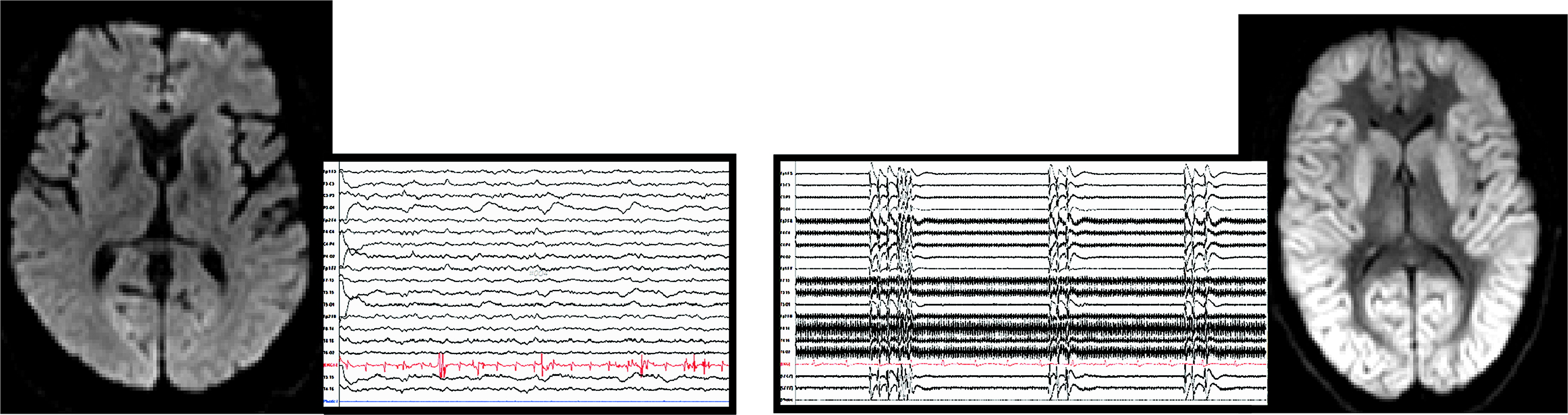
Several recent reviews support the potential of MRI to improve the prediction of poor neurologic outcomes in patients who remain comatose after CA.
1–5 Diffusion (diffusion-weighted imaging [DWI] or diffusion-tensor imaging [DTI]) and fluid-attenuated inversion recovery (FLAIR) sequences are both of value during the acute stage, although DWI or DTI may provide visualization of some areas of injury earlier than FLAIR. Poor outcome (death, vegetative state, or severe disability) is associated with more widespread and severe areas of MRI abnormality (
Figure 1). However, the evidence is still deemed weak because of study limitations (e.g., small sample sizes, retrospective design, or lack of standardization for multiple variables, including timing of imaging, quantification of injury, timing, and method of outcome assessment).
1–5 Two recent prospective studies utilized a standardized visual scoring system (21 predefined cortical and subcortical brain regions; from 0=normal to 4=severely abnormal) for rating MRI changes consistent with acute global hypoxic-ischemic brain injury.
28,29 Although these studies differed considerably in multiple variables, including sample size (68 versus 19), time of imaging (median, 77 hours versus <6 hours), and outcome assessment (6 months versus hospital discharge day), both studies reported that the summed score for cortical gray matter regions provided excellent prediction of poor outcome (death or vegetative state). The focus in almost all of these studies was on patients in a prolonged coma after CA, limiting applicability to patients with less severe brain injuries.
Cognitive Outcomes After CA
A systematic review of studies (1980–2006) assessing cognitive function at a minimum of 3 months after CA reported that the frequency of cognitive impairment ranged from 6% to 100%.
30 The only factor consistently associated with a better outcome was short time to awakening (e.g., awake on admission, or in a brief coma). The authors identified multiple differences in methodology that likely contributed to variability across studies (e.g., subject selection, small samples, and timing and methods of cognitive assessment). The three strongest studies (e.g., prospective, standard neuropsychological measures administered at least 6 months after CA) all concluded that cognitive problems occurred in approximately one-half (42%, 48%, and 50%, respectively) of CA survivors.
30 Recent prospective studies have explored specific aspects of cognitive outcome after CA.
Measures can vary considerably in the sensitivity to cognitive function, making comparison across studies difficult. Multiple studies have found that the most commonly utilized functional assessments at hospital discharge (the cerebral performance category [CPC] and the overall performance category [OPC]) were not sensitive predictors of outcome, because both improvements and decrements were noted at follow-up for patients with discharge ratings of 1 (good cerebral performance, able to lead a normal life), 2 (moderate disability, sufficient cerebral function for independent activities of daily life), and 3 (severe cerebral disability, dependent on others for daily support).
14,15,31,32 In two studies,
15,32 cognitive screening tools (e.g., the Mini-Mental State Examination and the Telephone Interview for Cognitive Status) were more sensitive to the presence of cognitive impairments than the CPC, because both reported the presence of cognitive impairments at follow-up (1 and 6 months; median, 20 months) in patients with a CPC rating of 1. A study that obtained standard neuropsychological measures later after the CA (median, 7.8 years) reported impaired long-term memory and learning efficiency compared with community norms, although most patients were cognitively intact based on their Mini-Mental State Examination scores.
14 All but one patient had received an OPC rating of 1 at hospital discharge.
More recent studies disagree on whether a short time to awakening is consistently associated with better cognitive outcome. A study that included all levels of severity found similar memory deficits at 1 month in patients who were awake (N=7) and in patients who were in a coma (N=26) on admission to the hospital.
33 In addition, patients with CPC ratings of 1 (N=22) and 2 (N=11) at 1 month were impaired on several measures of memory compared with controls (matched for age, sex, and educational level) (N=33). The authors noted that these results contradicted the common assumption that patients who awaken soon after resuscitation will have fewer cognitive impairments than patients with a more prolonged coma.
33 Similarly, a study that compared patients who awakened early (N=88) with patients who received therapeutic hypothermia (N=156) reported comparable proportions of patients scoring in the normal range on screening measures for functional independence (88% versus 78%) and cognition (both 83%) at 6–12 months.
34 By contrast, several studies reported that a shorter-duration coma was associated with better cognitive performance at times ranging from 3 to 12 months.
10,35,36 A study obtaining standard neuropsychological measures at 3 months (N=45) reported that both a shorter coma duration and use of therapeutic hypothermia were associated with better cognitive performance.
36 Overall, 56% (25 of 45) of patients scored in the average range on all tests, 31% (14 of 45) were impaired on one or two tests, and 13% (six of 45) were impaired on three or more tests.
36 The authors noted that these results exceeded the rate expected for the general population (19% impaired on one or more tests). The domains most affected were fine motor control, memory, attention, and executive functions. A set of studies utilizing standard neuropsychological measures compared patients recovering from CA (N=30) who had experienced a coma (coma duration >1 day, responsive but confused at 1 week) with patients recovering from any other acute coronary syndrome (N=30) at 3 and 12 months.
10,35 The performance of the coronary syndromes group was essentially normal in all domains tested (memory, executive, semantic, perceptual, and psychomotor) at both times. By contrast, one-third (10 of 30) of the CA group was severely impaired (all domains) and two-thirds (18 of 30) had modest deficits (primarily in the memory and psychomotor domains), with longer coma duration associated with greater impairments. Contrary to expectations, little functional improvement was seen between 3 and 12 months.
10 Although another recent study reported that the proportion of patients with clear cognitive dysfunction (deficits on neuropsychological measures in three or more domains) decreased with time (57% in the hospital, 47% at 3 months, 36% at 6 months, and 14% at 12 months), the data for the group that survived to the final time point were not reported at each interval.
37 The authors of this study emphasized that the presence of cognitive impairment in a substantial portion of survivors many months after CA indicates the need for neurorehabilitation.
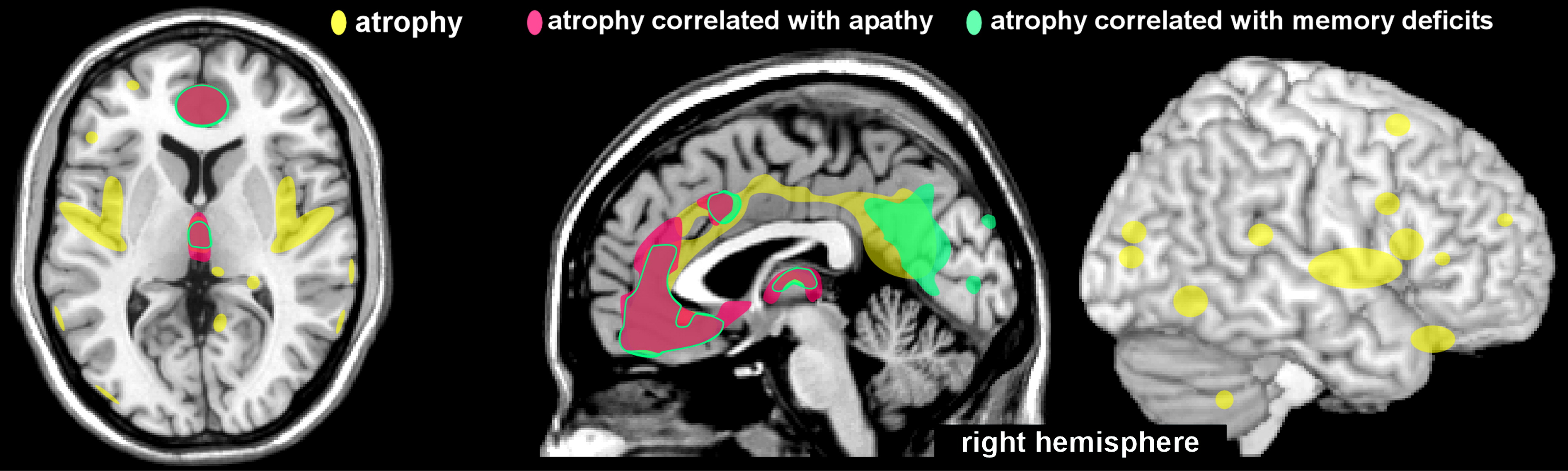
Only a few studies have investigated the anatomic correlates of observed cognitive deficits. A case-control study utilized voxel-based morphometry to identify areas of brain atrophy after recovery from CA.
6 Neuropsychological measures and MRI scans were obtained from survivors of CA (N=12) in the chronic stage (mean, 15 months; range, 4–62 months). Patients with macroscopic lesions were excluded, and the median duration of coma was 1 day (range, 0–14 days). Compared with controls (individually matched for age and sex), the patients exhibited atrophy (gray matter volume decrease) in multiple areas (e.g., anterior and posterior cingulate, medial parietal and insular cortices, posterior hippocampus, and thalamus) in the group-wise comparison (
Figure 2). The degree of atrophy in specific regions correlated with specific neuropsychological impairments (
Figure 2). Atrophy in the anterior cingulate cortex and dorsomedial thalamus correlated with both memory and motivational (drive) deficits.
6 Atrophy in an area encompassing the retrosplenial cingulate cortex and part of the precuneus correlated with severity of memory deficits.
6 Although atrophy was noted in the hippocampus (limited to the posterior region on the left), this did not correlate significantly with memory performance. These findings are in agreement with an earlier study,
7 which concluded that memory impairments were associated with diffuse global cerebral atrophy, rather than hippocampal reductions.
Psychosocial Outcomes and Quality of Life After CA
Two recent systematic reviews focused on different aspects of more patient-centered outcome measures in survivors of CA. One review concentrated on studies that included some assessment of quality of life at a minimum of 6 months after CA.
38 Although the majority of studies either reported good quality of life (46 of 70) or were neutral (17 of 70), a few studies (seven of 70) reported poor quality of life.
38 The authors commented on the methodological heterogeneity of the reviewed studies, as well as the need for standardized approaches to assessing quality of life. The other review identified studies that included standard measures of psychological distress at any time after CA.
39 Rates varied considerably across studies for all conditions, with 14%−45% for depression, 13%−61% for anxiety, and 19%−27% for posttraumatic stress disorder. The authors commented that these results indicate routine screening is warranted to identify patients who would benefit from treatment.
Several recent prospective studies have utilized standard measures of psychosocial functioning and quality of life in survivors of CA. Studies that included a group of patients who experienced coma (e.g., received therapeutic hypothermia, coma duration of 12 hours to 7 days), with at least 6 months of follow-up, reported that the majority of patients rated their quality of life as good on the global measure (56%−70%) and mental domain (89%−92%).
9,32,34 A lower proportion (49%−62%) rated their physical quality of life as good.
9,34 The rates of clinically significant levels of psychiatric symptoms varied considerably (depression, 0%−33%; anxiety, 8%−52%).
9,10,40 Compared with patients recovering from other acute coronary syndromes, survivors of CA reported lower quality of life in the psychological and social domains but not in the physical domain.
10 Two studies comparing survivors of CA by some measure of initial severity (significant coma versus awakening early, or requirement versus no requirement of therapeutic hypothermia), with at least 6 months of follow-up, found that relatively similar proportions of each severity group rated their quality of life as good (global, mental domain, and physical domain).
34,40 By contrast, the study that included measures of psychiatric symptoms found that the group requiring therapeutic hypothermia reported higher rates of clinically significant depression (33% versus 7%), anxiety (52% versus 21%), and posttraumatic stress (22% versus 7%).
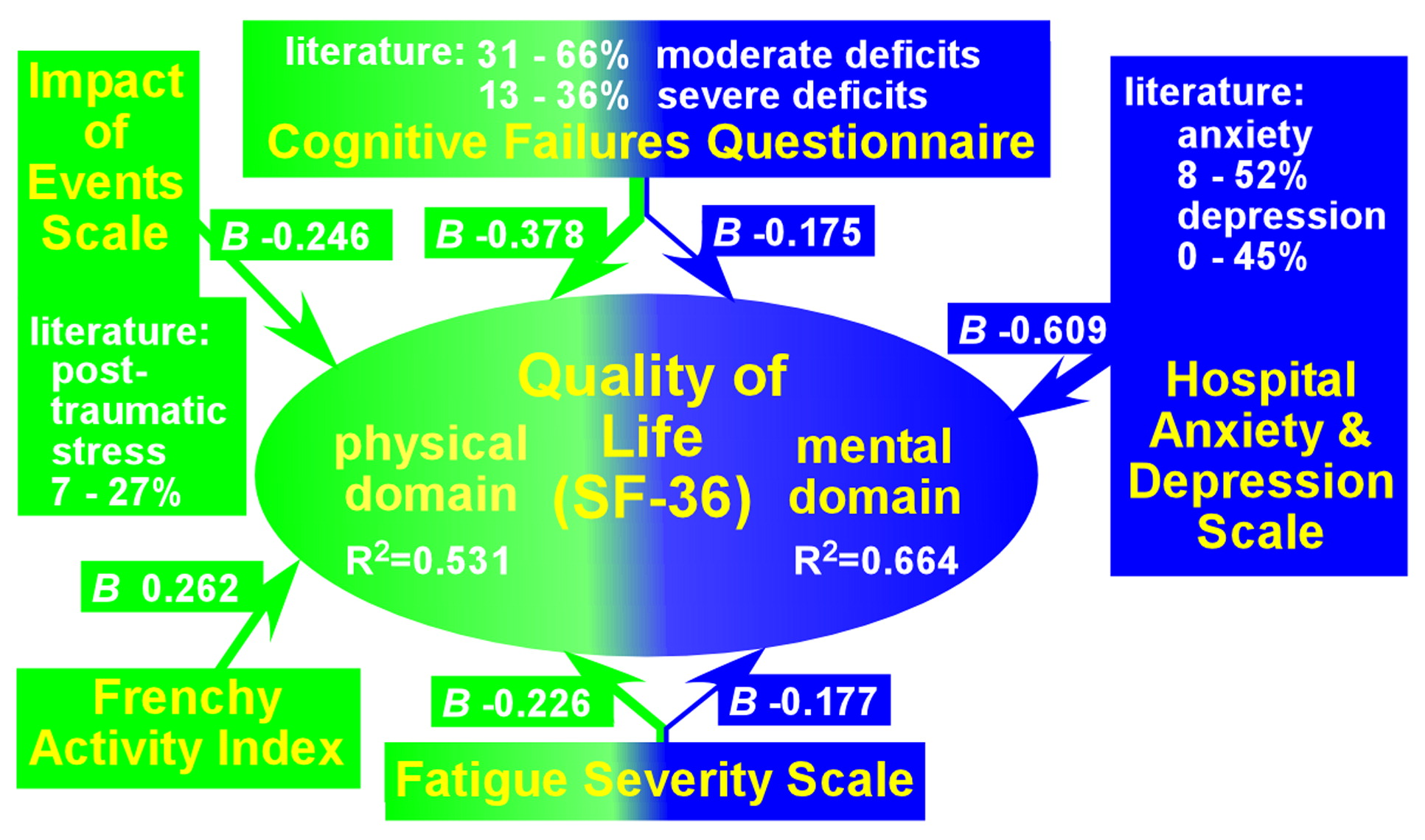
40 Several studies have reported correlations between lower ratings on quality-of-life measures and dysfunctions in specific domains (including cognition, memory, anxiety, depression, posttraumatic stress, fatigue, and activities of daily living) (
Figure 3).
8–10 The authors of one study noted that their results support treating survivors of CA similarly to other brain-injured patients, and the authors suggested that identifying the domains affected in a specific patient (e.g., cognitive, fatigue, and psychiatric) could guide the design of an appropriate interdisciplinary rehabilitation program.
8Screening and Treatment
The need for psychosocial and cognitive screening and early intervention is clearly indicated in the post-CA population.
39 Screening for psychosocial problems (e.g., psychiatric symptoms, activities of daily living, quality of life, and fatigue) is easily implemented because many validated self-report instruments are available. The International Liaison Committee on Resuscitation, Cardiac Arrest, and Cardiopulmonary Resuscitation recommended using the CPC on hospital discharge, followed by assessments of neurologic function at 6 months and 1 year after CA.
41,42 As discussed above, the CPC rating is not sensitive to the presence of cognitive impairments. Although many studies support the value of standard neuropsychological testing, this requires considerable patient time and the availability of specially trained personnel. Two recent studies demonstrated the feasibility of using a computer-based battery of neuropsychological tests to quickly identify domains of cognitive impairment in survivors of CA.
43,44 One study focused specifically on survivors of CA with a CPC rating of 1 at hospital discharge (N=29).
43 None of the patients were in the acute stage of recovery (mean, 15.2 months after CA; range, 4–27 months). The 11 administered tests in their validated cognitive battery assessed the cognitive domains of executive function (abstraction and mental flexibility, attention, working memory), episodic memory (immediate and delayed for verbal, facial, and spatial), complex cognition (language reasoning, spatial processing), social cognition (emotion identification), and processing speed (sensorimotor speed, motor speed). Compared with controls (matched for age, sex, race, and demographics), the patient group scored within the normal range for accuracy and speed in most domains. The most profound deficits were in working memory accuracy (mean z score −1.12; all scores >1 SD below the mean). Deficits in spatial memory speed were also common (mean z score −1.26; 21 of 29 z scores ≤−2). One-quarter (seven of 29) of patients were considered cognitively impaired (z score ≤−2 on two or more domains). The authors noted the importance of assessing both accuracy and speed, and they suggested that this approach may have value in longitudinal assessment of functioning, although they acknowledged the presence of major study limitations (e.g., small sample size and a lack of age-matched normative data for the complete battery that was used to assess patients).
43 The other study included survivors of CA with a CPC rating of either 1 or 2 at hospital discharge (N=30).
44 The median time after CA was 3.6 years. Five tests from the Cambridge Neuropsychological Test Automated Battery were administered to evaluate executive function and memory. Compared with the Cambridge Neuropsychological Test Automated Battery norms, 29% (eight of 28) of the patients were cognitively impaired (two of 10 z scores <−2 or three of 10 z scores <−1.5). The greatest impairment was in the domain of memory, with short-term memory most affected. As noted in both of these studies, a large number of survivors of CA could benefit from undergoing assessment for cognitive impairments. These studies support the potential of computer-administered cognitive testing as a practical tool for screening, at least in the later stages of recovery from CA.
The presence of impairments (cognitive, psychosocial, and/or psychiatric) in a substantial portion of CA survivors supports the need for targeted interventions.
45 Treatments for impairment after CA are primarily based on rehabilitation approaches used in other neurologic conditions such as traumatic brain injury or stroke.
45 Nonpharmacologic interventions include compensatory strategy development, behavioral adaptation, environmental modification, and formal cognitive rehabilitation.
45 Pharmacologic strategies include agents that augment cerebral catecholaminergic function and/or cholinergic function. A neurologically focused early intervention program for patients with CA was positively evaluated overall by the nurses performing the intervention as well as by the patients and caregivers.
46 The intervention consisted of cognitive and emotional screening, dissemination of information and support, promotion of self-management, and referral for specialty services if deemed appropriate. Going forward, recommendations included administration of formal screening instruments to all patients with CA and provision of at least two intervention sessions.
46