There are many mysteries associated with Alzheimer’s disease, but perhaps one of the most fundamental revolves around the amyloid beta (Aβ) protein that forms the plaques associated with the disease. What is this protein’s normal purpose?
No one knows for sure, though a traditional view is that Aβ is just a metabolic byproduct with no function. However, new research published May 25 in Science Translational Medicine has hinted that this protein may play a role in protecting the brain against microbial invaders. But in carrying out its short-term protective role, Aβ may be planting the seeds for long-term damage.
A team at the Genetics and Aging Research Unit at Harvard Medical School used three different model systems—mouse, worm, and cultured brain cells—to demonstrate that Aβ can provide resistance to infection from both bacteria (Salmonella) and infectious yeast (Candida) and promote increased survival.
For example, mice modified to contain about five times the normal levels of amyloid protein survived for nearly 96 hours after injection of Salmonella, while mice without the high levels of amyloid protein died before 72 hours and mice with no amyloid died before 60 hours.
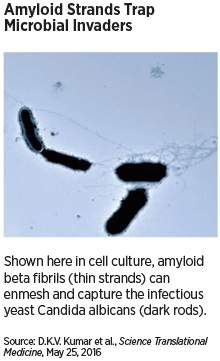
The amyloids worked by clustering around the invading microbes and attaching to carbohydrates on the cell surface; the individual protein pieces then binded to other amyloid fragments, forming thin extensions that found their way to other microbe-bound amyloids. The end result was a fibrous amyloid net that trapped the microbes and rendered them powerless.
It is this same process of fibrillization that eventually creates the large, insoluble clumps of Aβ that are found in the brains of people with Alzheimer’s—suggesting that this same infection-fighting mechanism might be involved in the disease process.
The process by which Aβ plaque formation becomes widespread is unclear, though. It may be a gradual accumulation over time and multiple infections, the authors suggested, or it could also arise from an abnormal activation of the protein triggered by inflammation, akin to an autoimmune response.
The authors did caution that other basic questions still need to be addressed before too many conclusions are made. For one, their work presented evidence that Aβ has biological activity, but for the purpose of research, they conducted most of their work in animals genetically modified to produce either excess amyloid or no amyloid. So how important Aβ is in a normal immune response remains to be seen.
Nevertheless, these intriguing findings may require a re-examination of the maligned Aβ molecule. And while these experiments were carried out in model systems, the association between infection and Alzheimer’s in humans has been the subject of previous investigations. For example, a study published recently in the journal
Alzheimer’s Disease and Associated Disorders provided some of the first causal evidence, showing that people chronically infected with Cytomegalovirus or Herpes virus had a greater rate of cognitive decline over time (
Psychiatric News, March 4).
This study was supported by the National Institute of Allergy and Infectious Diseases, the Cure Alzheimer’s Fund, and the Helmsley Charitable Trust. ■
An abstract of “Amyloid-β Peptide Protects Against Microbial Infection in Mouse and Worm Models of Alzheimer’s Disease” can be accessed
here.