Could an FDA database that compiles reports of negative side effects of approved medications point researchers to medications that could be used off-label to treat symptoms of depression? Researchers at the University of California, San Diego (UCSD), believe the answer may be yes, according to an article appearing May 3 in Scientific Reports.
“Although FAERS [the FDA Adverse Event Reporting System] was originally intended to track frequent adverse events, with sufficient … data, it can also be used to track the beneficial outcomes indirectly through monitoring reductions of related complaint frequencies,” wrote Ruben Abagyan, Ph.D., a professor in UCSD’s Skaggs School of Pharmacy and Pharmaceutical Sciences, and colleagues. The team reported that data contained in this tracking system provided additional evidence of the antidepressant effects of the anesthetic ketamine.
While several trials have demonstrated the acute efficacy of ketamine in treating treatment-resistant depression, bipolar depression, and more, most of these trials have had a low number of participants. For a larger sample of patients, Abagyan’s team scoured over 8 million patient reports entered in FAERS between 2004 and 2016 and identified 41,337 patients taking ketamine (either alone or in combination with other agents) for pain and 238,516 patients taking other combinations of medications for pain.
They found that the incidence of reported depression was about 67 percent less among the ketamine group compared with people taking other pain relievers. Pain reports were also significantly lower in patients taking ketamine.
“This study extends small-scale clinical evidence that ketamine can be used to alleviate depression and provides needed solid statistical support for wider clinical applications,” Abagyan said in a press release.
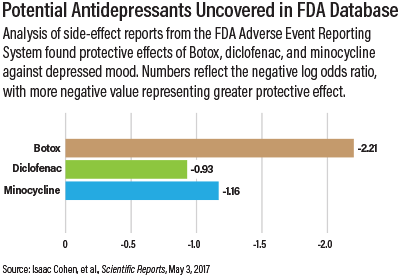
Ketamine was not the only drug to show some antidepressant action in the FAERS analysis; people getting facial Botox injections, the non-steroidal anti-inflammatory agent (NSAID) diclofenac, and minocycline also had reduced depression symptoms. Botox’s effects may be tied to the fact that facial nerves are connected to brain regions regulating mood and emotion. Diclofenac and minocycline, meanwhile, both display anti-inflammatory properties, and research suggests that inflammation contributes to depression.
To determine whether the potential antidepressant effects of NSAIDs were influencing the differences between patients taking ketamine and other pain medications, Abagyan and his team reanalyzed the groups, excluding people in either group who took NSAIDs. Patients taking ketamine reported depression 64 percent less often than those on other pain relievers.
“This study outlines a methodology for discovering off-label pharmacology of existing approved drugs. This method can be applied to other indications and may reveal new important uses of already approved drugs, providing reliable justification for new indications without large investments in additional clinical trials,” Abagyan and colleagues concluded in their report.
The Skaggs School of Pharmacy and Pharmaceutical Sciences funded this research. ■
“Population Scale Data Reveals the Antidepressant Effects of Ketamine and Other Therapeutics Approved for Non-Psychiatric Indications” can be accessed
here.