Shortened Positive and Negative Symptom Scale as an Alternate Clinical Endpoint for Acute Schizophrenia Trials: Analysis from the US Food & Drug Administration
Abstract
Objective
Design
Results
Conclusions
HIGHLIGHTS
Methods
Data Collection
| Characteristic | Number | % |
|---|---|---|
| Number of trials | 32 | |
| 6‐week trials | 28 | 87.5 |
| 4‐week trials | 4 | 12.5 |
| Number of treatment arms (including active control) versus placebo | 86 | |
| Total number of subjects | 14,219 | |
| Subjects randomized to placebo | 3533 | 24.8 |
| Subjects randomized to drug treatment (including active control) | 10,686 | 75.2 |
| Demographics | Mean | (SD) or (%) |
|---|---|---|
| Age, years | 39.0 | 11.0 |
| Females | 4414 | 31.0% |
| Caucasian | 7183 | 51.5% |
| African Americans | 4346 | 30.5% |
| Asians + others | 2690 | 18.0% |
| Baseline total PANSS | 94.4 | 13.6 |
Derivation of mPANSS
Concordance Analysis
Sample Size Estimation
Results
Derivation of mPANSS
Summary of baseline PANSS item scores
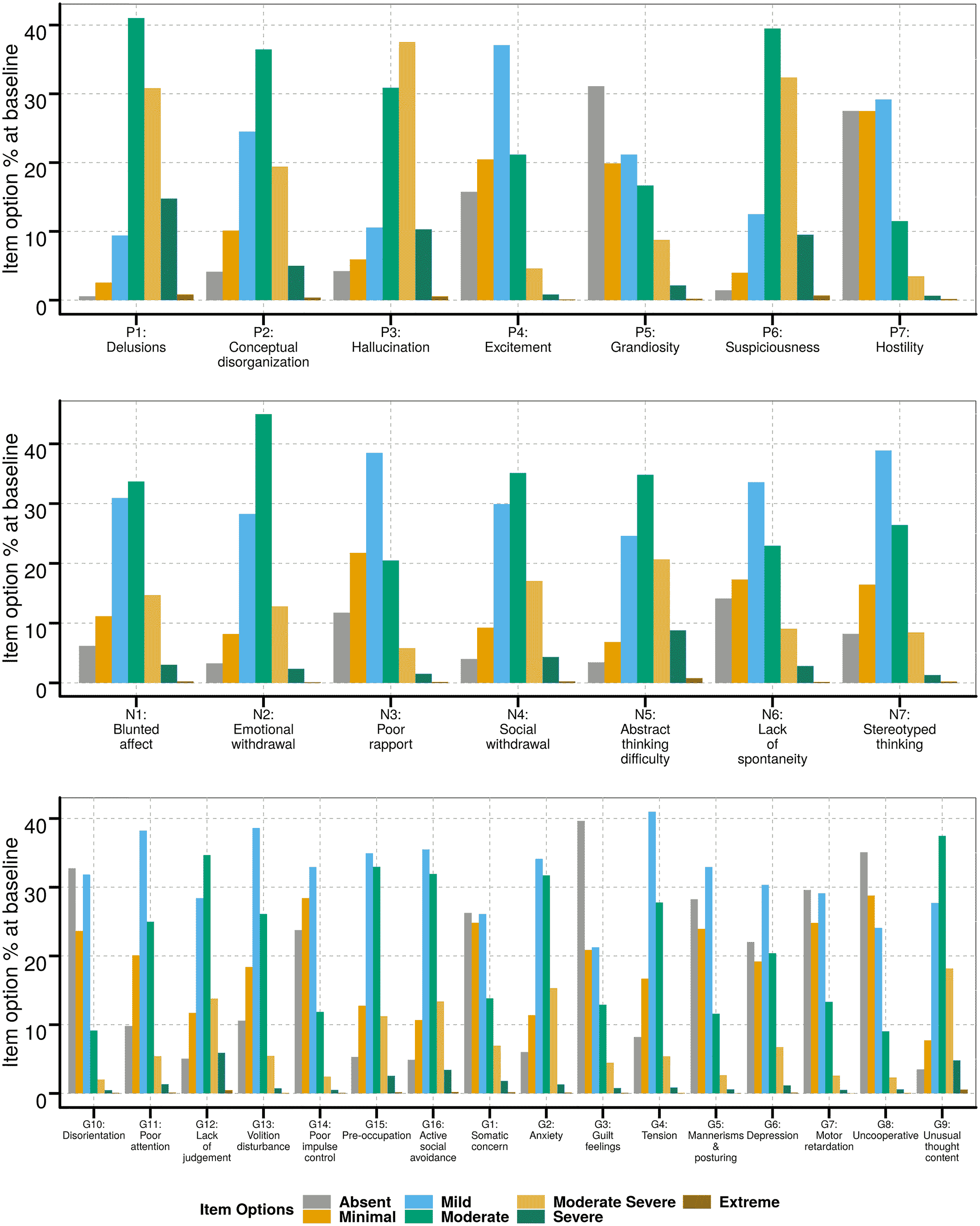
Item response analysis to derive modified PANSS
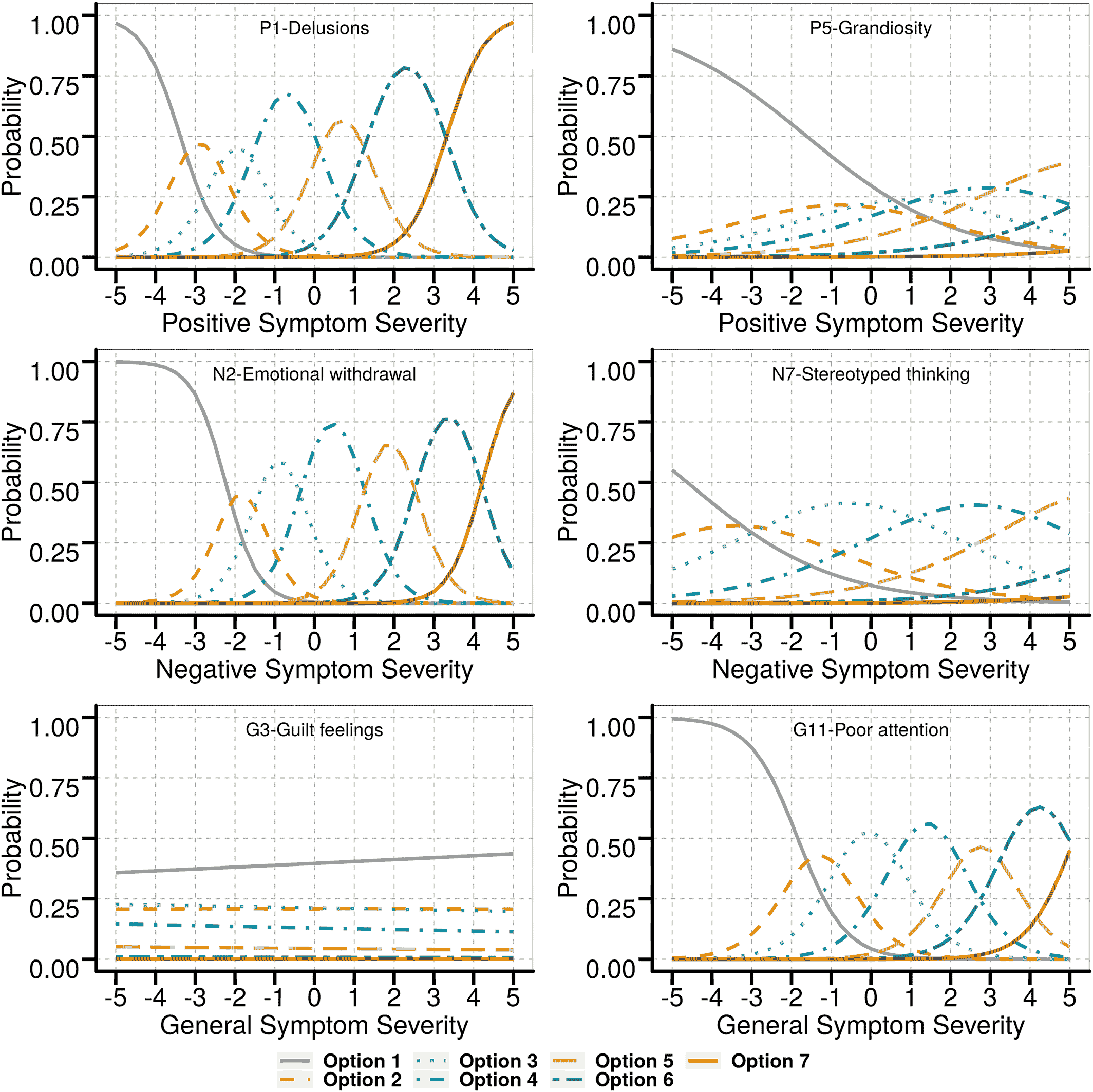
| Item | Criterion 1 | Criterion 2 | Criterion 3 | Criterion 4 | Criterion 5 | Evaluation |
|---|---|---|---|---|---|---|
| Positive subscale items | ||||||
| P1: Delusions | Yes | Yes | Yes | Yes | Yes | Very good |
| P2:Conceptual disorganization | Some | No | Some | Yes | No | Poor |
| P3: Hallucination | Yes | Some | Yes | Yes | Yes | Good |
| P4: Excitement | Some | Some | Yes | Yes | Yes | Good |
| P5: Grandiosity | Some | No | Some | Yes | Yes | Poor |
| P6: Suspiciousness | Yes | Yes | Yes | Yes | Yes | Very good |
| P7: Hostility | Yes | Some | Yes | Yes | Yes | Good |
| Negative subscale items | ||||||
| N1: Blunted effect | Yes | Yes | Yes | Yes | Yes | Very good |
| N2: Emotional withdrawal | Yes | Yes | Yes | Yes | Yes | Very good |
| N3: Poor rapport | Yes | Yes | Yes | Yes | Yes | Very good |
| N4: Passive social withdrawal | Yes | Yes | Yes | Yes | Yes | Very good |
| N5: Difficulty in abstract thinking | Yes | Some | Yes | Yes | Yes | Good |
| N6: Lack of spontaneity | Yes | Yes | Yes | Yes | Yes | Very good |
| N7: Stereotyped thinking | Some | Some | Some | Yes | Yes | Poor |
| General psychopathology subscale items | ||||||
| G1: Somatic concern | Some | No | Some | No | No | Poor |
| G2: Anxiety | Some | No | Some | No | No | Poor |
| G3: Guilt feelings | No | No | No | No | No | Poor |
| G4: Tension | Yes | Some | Yes | Yes | Yes | Good |
| G5: Mannerisms & posturing | Some | Some | Some | Yes | Yes | Poor |
| G6: Depression | Some | No | Some | No | No | Poor |
| G7: Motor retardation | Some | Some | Some | Yes | No | Poor |
| G8: Uncooperative | Yes | Yes | Yes | Yes | Yes | Very good |
| G9: Unusual thought content | Yes | Some | Yes | Yes | Yes | Good |
| G10: Disorientation | Some | Some | Some | Yes | Yes | Poor |
| G11: Poor attention | Yes | Yes | Yes | Yes | Yes | Very good |
| G12: Lack of judgment | Yes | Yes | Yes | Yes | Yes | Very good |
| G13: Disturbance volition | Yes | Yes | Yes | Yes | Yes | Very good |
| G14: Poor impulse control | Some | Some | Some | Yes | Yes | Poor |
| G15: Pre‐occupation | Yes | Yes | Yes | Yes | Yes | Very good |
| G16: Social avoidance | Yes | Some | Yes | Yes | Yes | Good |
Concordance Analysis
| Concordance rate (6‐week mPANSS vs. 6‐week total PANSS) | |
|---|---|
| Overall | 97.6% (84/86) [false negative: 1, false positive:1] |
| By trial design | |
| Fixed | 97.1% |
| Flexible | 100% |
| By drug | |
| 1 | 100% |
| 2 | 100% |
| 3 | 100% |
| 4 | 100% |
| 5 | 100% |
| 6 | 100% |
| 7 | 90% [false positive:1] |
| 8 | 92.3% [false negative: 1] |
Sample Size Considerations
Discussion
Footnotes
Supplementary Material
- Download
- 43.58 KB
- View/Download
- 6.02 KB
- View/Download
- 18.29 KB
- View/Download
- 17.89 KB
- View/Download
- 20.22 KB
- View/Download
- 20.21 KB
References
REFERENCES
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
