Delirium is a relatively common illness that occurs in approximately 11%–42% of hospitalizations and seriously affects mortality, functional outcomes, length of hospital stay, and institutional admission (
1). Although many cases of delirium resolve within a few days, approximately 20% of the cases last for weeks or months (
2). Therefore, identifying factors associated with poor outcomes in patients with delirium is important for predicting prognosis (
3).
Previous studies have shown that severe delirium (e.g., severe cases of sleep‐wake rhythm disturbances, perceptual disturbances, delusions, cognitive impairment, and psychomotor activity) has a poor prognosis, including high mortality (
4,
5). However, the general delirium rating scales (i.e., Delirium Rating Scale Revised 98 [DRS‐R] and Memorial Delirium Assessment Scale [MDAS]) used in these studies are difficult to use in a clinical setting because of administration time and prerequisite training (
6). Bellelli et al. developed the 4 “A”s test, also known as the 4AT, to address the shortcomings of the existing delirium assessment tools (
6). The 4AT is a tool that does not require specialized training and consists of only four components: awareness, cognitive functions, attention, and a significant change or fluctuation in the patient's mental or alertness condition. The tool has high sensitivity and specificity for predicting mortality in patients with delirium (
7). A study examining the relationship between severity measured by the 4AT and prognosis showed higher mortality rates among severe cases, indicating that cognitive function may be associated with poor prognosis in patients with delirium (
8).
The 4AT includes subjective items, a concern raised in previous studies, as owing to its simplicity (
8), it can reduce the screening sensitivity in a busy clinical environment, such as an intensive care unit (
9). Therefore, a separate tool, less susceptible to subjectivity, is necessary to evaluate the prognosis of delirium in a busy clinical setting.
Regarding objectivity, a small‐size bispectral electroencephalography (EEG) device was recently developed, with reports indicating that slower EEG activity correlates with higher delirium severity and mortality (
10). However, such specialized examination and analysis equipment are not widely available in general hospitals.
In this study, we developed an easy and objective cognitive function measurement scale that could predict the prognosis and mortality related to delirium.
Methods
Participants
This retrospective study was conducted at Nihaonkai General Hospital, an acute general hospital with 630 beds and without a psychiatry ward in the Shonai region of Yamagata Prefecture, Japan. The study was conducted according to the Declaration of Helsinki, and was approved by the Institutional Review Board of Nihonkai General Hospital (approval number: 00239). Owing to the retrospective study design, the requirement for obtaining informed consent from the patients was waived.
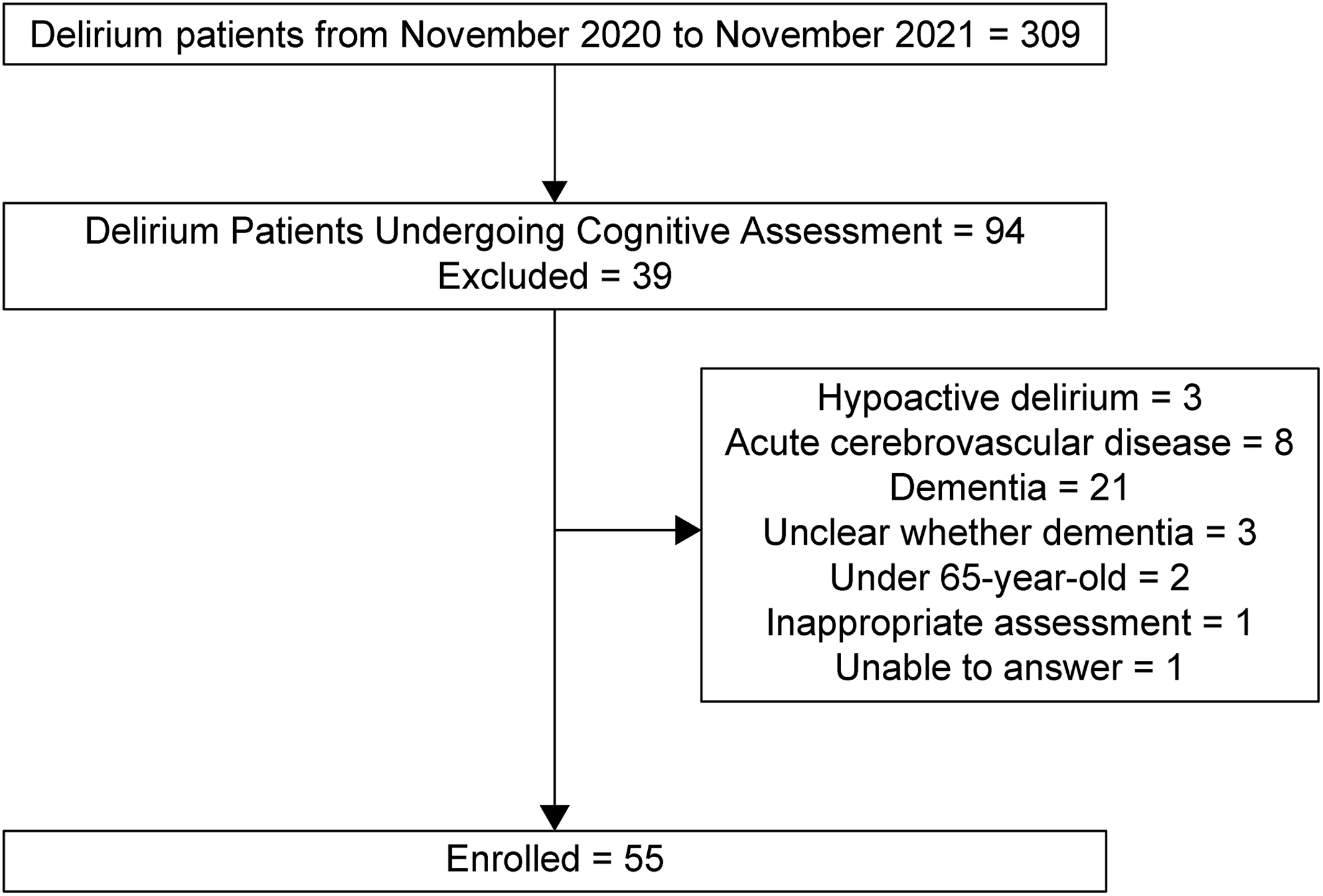
Three hundred nine patients aged ≥65 years who met with our psychiatry consultation team between November 2020 and November 2021 were diagnosed with delirium by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (
11). Two psychiatrists (YS and SK) reviewed the patients' medical records.
Our inclusion criteria required that information on orientation related to place, time, and attention, which was assessed at the first consultation, was included in the medical records. We excluded patients diagnosed with hypoactive delirium because it is known to have a poorer prognosis than the other motor subtype of delirium (
12). In addition, drug‐induced delirium, alcohol withdrawal syndrome, acute cerebrovascular disease, dementia, or those who could not communicate because of sedation were also excluded to remove other types of cognitive function impairment. Patients with dementia were defined as individuals with clinically diagnosed dementia or those who underwent routine family interviews to detect dementia before hospitalization. In total, 55 patients with delirium were identified for this study (
Figure 1). The primary outcome was death within 6 months after the first visit.
Assessment of Cognitive Function
Since the purpose of this study was to identify factors associated with the prognosis of delirium quickly and objectively, we referred to the simplest of the 4AT among existing delirium assessment tools. We focused on time and place orientation and attention, which were objective evaluation items among 4AT.
The reviewing psychiatrists extracted data regarding time and place orientations and attention (aspects examined at the bedside during the first visit from 10 a.m. to 4 p.m. by the consulting psychiatry team). Only items corresponding to the subitems of the revised Hasegawa Dementia Scale (HDS‐R) (
13,
14), a commonly used test for screening dementia in Japan, were evaluated. Patients were assessed for the following:
1.
Orientation
a.
Date: considered correct only if both month and day are correct
b.
Location: correct answer only if the hospital name is correct
2.
Attention
b.
Four‐digit span reverse (not performed if the three‐digit span reverse is incorrect)
c.
Calculation 1 (subtract 7 from 100)
d.
Calculation 2 (subtract another 7 from the answer of calculation 1; not performed if calculation 1 is incorrect)
The correct answer for each item was set at one point, and in total, six points were evaluated. The average score for the 55 participants was 2.82 ± 1.61. As such, the demarcation point for cognitive function at the first visit was set to 2/3, where a score of ≤2 was considered low cognitive function (LCF), and a score of ≥3 was considered high cognitive function (HCF). The patients were categorized into two cognitive function groups, HCF and LCF, based on the average scores.
Assessment of Severity of Delirium
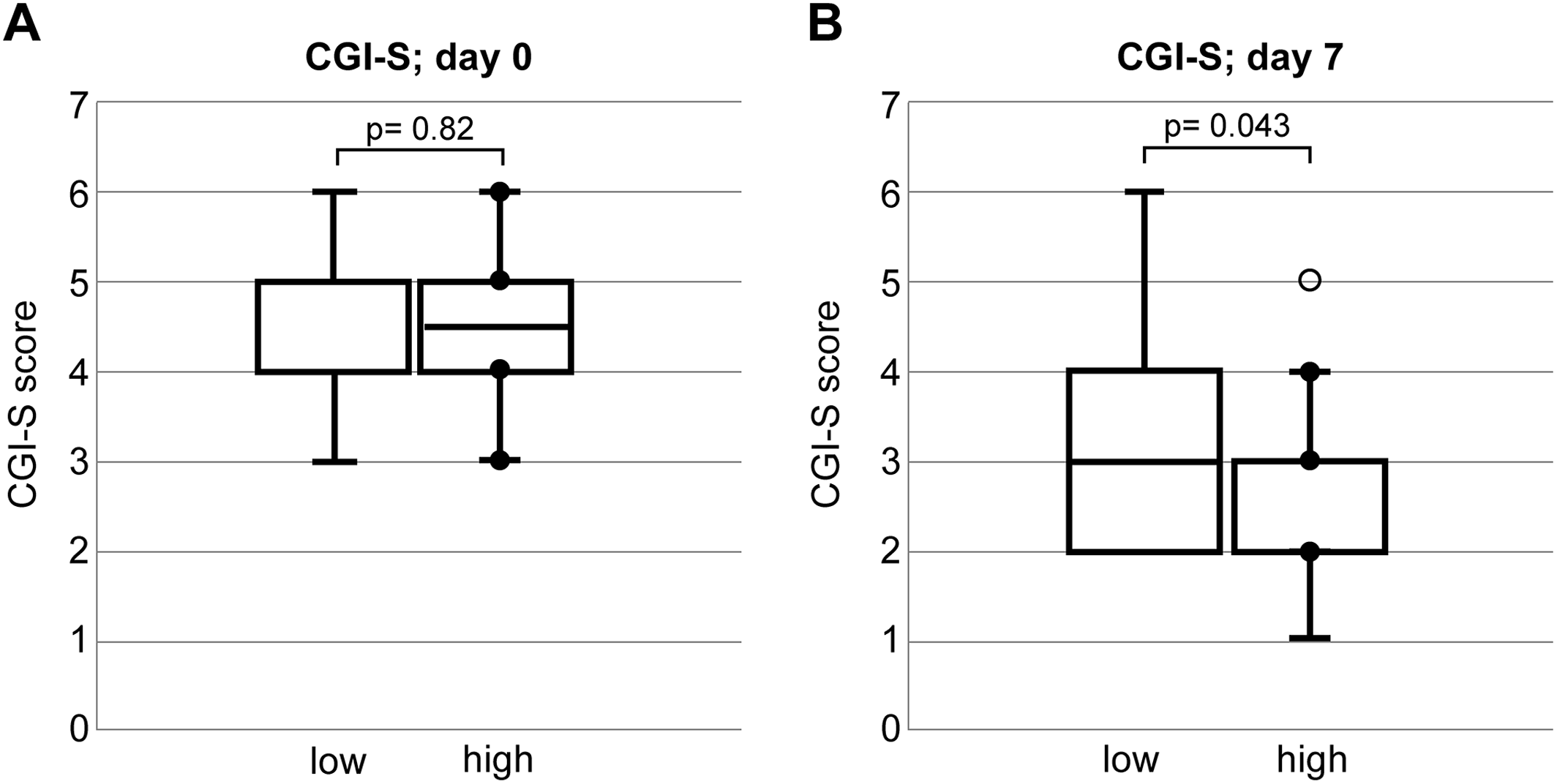
The severity of delirium at the first visit and after 1 week of treatment was evaluated using Clinical Global Impressions‐Severity scale (CGI‐S; Day 0 and Day 7) and rated on the following seven‐point scale: 1 = normal, not ill; 2 = borderline mentally ill; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill; and 7 = among the most extremely ill patients (
15). The CGI‐S provides an overall clinician‐determined summary measure that considers all available information, including knowledge of the patient's history, psychosocial circumstances, symptoms, behavior, and the impact of the symptoms on the patient's ability to function (
16). The CGI‐S score was determined by an independent review of the medical record by three psychiatrists (TN, TT, and RK). If there was disagreement regarding scoring, the three evaluators discussed it until they reached a consensus.
Statistical Methods and Analysis
Student's
t‐test and chi‐square tests were used to compare the clinical and demographic data between the HCF and LCF groups. Some CGI‐S scores were compared using the Mann–Whitney
U test. Univariate and multivariate logistic regression analyses were used to calculate the odds ratio (OR) and 95% confidence intervals (CIs) for mortality after 6 months as a function of cognitive function. The multivariable model was adjusted for age, sex, therapeutic agent (hypnotics, atypical antipsychotics, and hypnotics + atypical antipsychotics + others), and age‐adjusted Charlson Comorbidity Index as a general index of comorbidity (
17). All statistical analyses were performed using SPSS version 28 for Windows (IBM, Armonk, NY, USA). A two‐sided
p‐value of <0.05 was considered statistically significant.
Discussion
Our findings revealed that the mortality rates after 6 months were 9.4% and 47.8% in the HCF and LCF groups, respectively. Multivariate analysis, adjusting for age, sex, therapeutic agents at the first visit, and type of physical illnesses, found that cognitive function at the first visit was independently associated with mortality at 6 months. In addition, the CGI‐S measured 7 days after the initial assessment was associated with a significantly poorer prognosis in the LCF group. These results show that assessing orientation and attention at the initial delirium intervention may predict improvement in delirium during hospitalization and mortality after 6 months.
A recent meta‐analysis on the components of delirium and mortality showed that altered arousal levels, attention deficit, and disorientation are associated with higher mortality (
3). Furthermore, the combination of attention deficit and disorientation in older patients with delirium is a stronger predictor of in‐hospital mortality (
18).
Several studies investigating the severity and prognosis of delirium used MDAS (
4,
19), DRS‐R‐98 (
20), and 4AT (
8). MDAS and DRS‐R‐98 are difficult to apply in a busy clinical practice because of the required time and training. All the assessment tools for delirium, even the 4AT, can be difficult for untrained physicians to use to correctly assess alertness levels and acute fluctuations (
8). Simple delirium assessments such as the Confusion Assessment Method (
21) and Nursing Delirium Screening Scale (
22) are used to assess delirium. However, they include evaluation items that may be influenced by the examiner's subjectivity. On the contrary, this study's simple and objective cognitive function evaluation using orientation and attention can be applied in a real‐world clinical setting because of its unambiguity, short administration time, and lack of additional training requirements.
Scales that rely on consultant psychiatrists are challenging to use as a shared evaluation method for the entire hospital. However, many types of medical staff, including nurses, can perform our simple cognitive assessment without consulting with a psychiatrist or having special training. The accuracy of the delirium prognosis prediction may be improved throughout the hospital by conducting this evaluation, and treatment strategies for the good and poor prognosis groups may be selected more efficiently and appropriately. For example, the group expected to make good progress would receive delirium treatment according to a predetermined protocol before consulting a psychiatrist. Similarly, the group expected to make poor progress would receive psychiatric consultation as early as possible. Hatta showed that suvorexant and ramelteon are effective in preventing delirium (
23). In this study, the administration of such drugs significantly reduced subsequent delirium in patients at high risk for delirium and even in those who developed delirium before consultation. A protocol for administering such medications before psychiatric consultation may result in adequate delirium management for patients delirium, especially those who are expected to improve favorably.
This study has several limitations. First, this study was a retrospective single‐center study of a small sample of patients. Therefore, multicenter prospective studies are required to validate our results. Second, we excluded patients with dementia to rule out the effects of cognitive dysfunction related to dementia in this study. In practice, delirium with dementia cannot be ignored in clinical practice, and these results may not be able to be generalized to that condition. Third, while the simple orientation and attentional assessment used in this study can help assess the prognosis of delirium, it should be noted that it is less specific for delirium screening (
6). Finally, this study targeted hyperactive delirium and excluded hypoactive delirium, although only three cases were excluded. Therefore, this could indicate that the screening protocol for hypoactive delirium in our hospital is not very effective. Further research including hypoactive delirium is needed, which is challenging to diagnose (
24).
This simple cognitive assessment without subjective evaluation items can be performed quickly by anyone, which may be helpful in busy clinical practices. Larger prospective studies are needed to confirm whether orientation and attention assessment can predict the prognosis of delirium.
In conclusion, this retrospective study showed that a simple cognitive assessment of attention and orientation might predict unfavorable outcomes, including mortality, in patients with delirium.