In the past, the treatment of depression was largely under the purview of psychiatric practice. However, the introduction of newer, ostensibly safer, and better-tolerated antidepressants has increasingly shifted the treatment of acute depression into the primary care environment over the past decade. Because some people are treated successfully in primary care, a treatment "sieve" is created, in which a higher proportion of treatment-resistant patients present for psychiatric care. Among these patients, full recovery is even more difficult to achieve.
The problem, then, is that many patients do not recover rapidly or completely, and the clinician is faced with a treatment decision for which the empirical literature offers little guidance. Although research data support the benefits of particular treatments, few studies have compared the effectiveness of various treatments. In this paper we provide clinicians with a method for evaluating residual symptoms of depression that is oriented toward better matching of symptoms with treatment.
Dimensions of mood
In this section we define the concept of mood and how dimensions of mood relate to anxiety and depressive disorders. On the basis of empirical findings and conceptualizations by Watson and colleagues (
14,
15) and other researchers (
16), we suggest that although these categories have distinctive features, they also have broad areas of symptomatic overlap. We present a dimensional rather than a categorical approach to understanding mood and anxiety disorders. An understanding of these dimensions of mood and their underlying physiological bases may be helpful for predicting responses to pharmacological intervention.
Highly complex systems of emotional response have been proposed previously (
17). However, when emotions are observed in the context of behavioral responses, a simpler, two-factor model emerges. Although described by many terms, these two emotional motivational systems can be described as appetitive, which involves approaches to rewarding stimuli, and defensive, which involves the behavioral management of threat (
17). When either appetitive or defensive behavioral responses are engaged, more basic arousal responses can also be activated.
As proposed by Lang and associates (
17), anxiety disorders can be seen as resulting from defensive motivational structures in the brain. For example, the emotional responses to exposure to phobic stimuli engage acute fear responses with attendant physiological arousal, such as tachycardia and tachypnea, that are similar to those seen in animals who are presented with a threatening stimulus from the environment.
Other disorders, such as generalized anxiety disorder, are less well connected to the emotional and behavioral responses to immediate threat. However, avoidance or behavioral inhibition can also be engaged as part of a conditioned fear response in which anxiety symptoms may be experienced in anticipation of exposure to threat (
18). As Lang and colleagues (
18) noted, "fear is generally held to be a reaction to an explicit threatening stimulus.… Anxiety is usually considered a more general state of distress, more long-lasting, prompted by less explicit or more generalized cues, involving physiological arousal but often without organized functional behavior."
More purely depressive symptoms involve an inhibition of emotional and physiological responses to rewarding stimuli. Thus a key component of depression is a relative reduction in approach behaviors toward otherwise rewarding environmental cues, such as food or a sexual partner. In fact, certain animal behavioral models of depression involve just such behaviors (
17,
18). Thus three emotional responses have emerged: acute physiological arousal characterized by panic or intense phobic fear, chronic anxiety reactions to anticipated threat, and emotional response to reward.
But is there a parallel in human psychopathology? Over the past 15 years or so, a body of research literature about the nature of mood regulation and mood disorders has emerged (
19,
20). Interest in this area has been partly driven by the observed overlap between anxiety and depression. There is substantial symptomatic and diagnostic comorbidity of anxiety and depressive disorders. In addition, they have a common response to treatments, including psychotherapy—for example, cognitive-behavioral therapy—and medication, such as selective serotonin reuptake inhibitors (SSRIs) (
14,
21). Recent research has shown that common, fundamental substrates of human emotion are engaged in both anxiety and depressive disorders.
This view is supported by phenomenological, genetic, and neurobiological data. Clearly substantial overlap exists in the diagnostic criteria for depressive and anxiety disorders (
16). However, there also is considerable aggregation of anxiety and depressive disorders, both at the individual level—that is, co-occurrence of anxiety and depressive disorders across a person's life span (
16)—and within families (
22,
23,
24,
25,
26).
In addition, the results of several studies indicate that generalized anxiety disorder and major depression have a common genetic diathesis and that differences between the two conditions may be determined by environmental rather than genetic factors (
22,
23,
24). For example, stressful life events have been shown to increase the likelihood of both anxiety and depressive symptoms, but multiple stressors over a short period appear to increase susceptibility to depression per se (
27). Finally, there is evidence of significant sharing of neurobiological factors, particularly hyperadrenergic and hypercortisolemic features (
28). Together, these data support the notion of a genetic and physiological continuum between anxiety and depression.
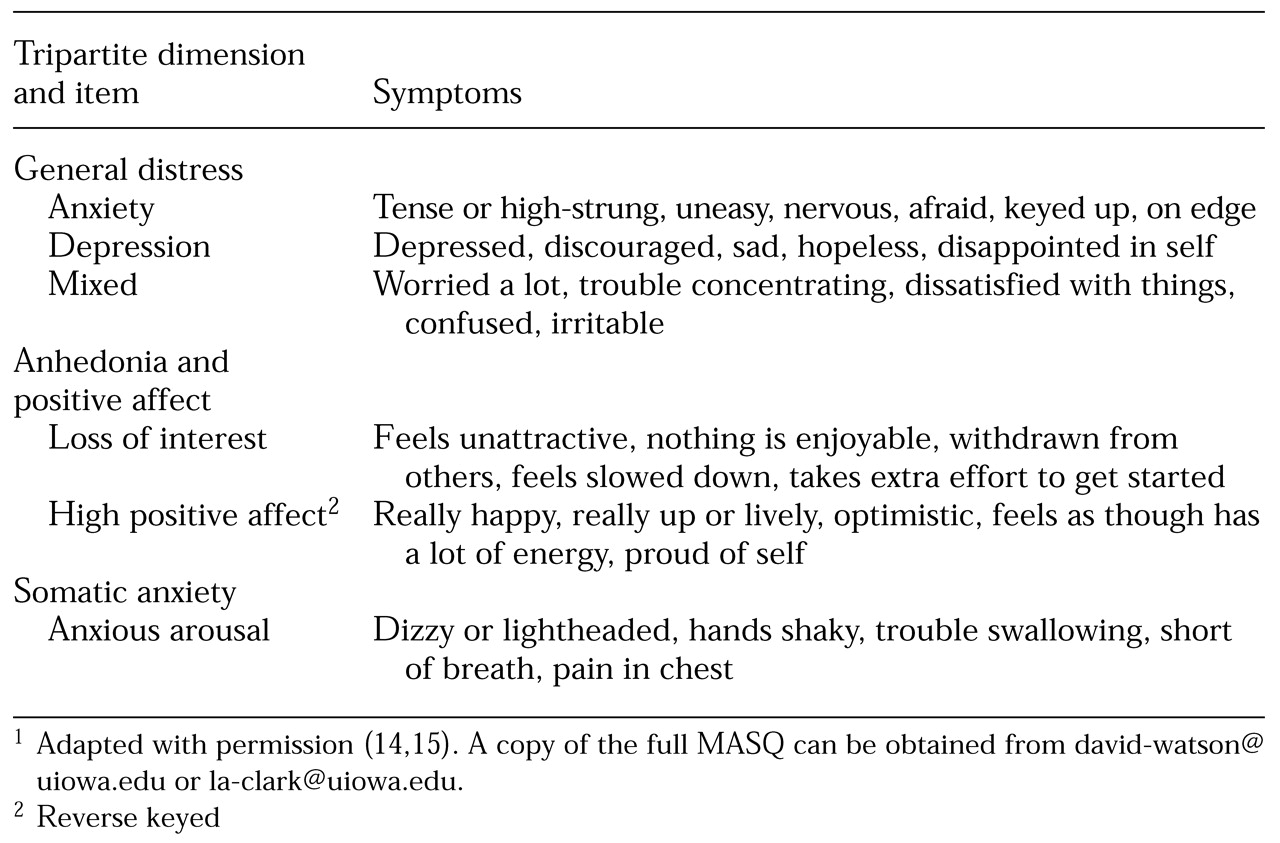
Research on human emotion has consistently demonstrated a tripartite model of mood and anxiety disorders, as outlined in
Table 1. Clark and Watson and their colleagues (
14,
15,
19,
20) developed the Mood and Anxiety Symptom Questionnaire (MASQ) to assess the higher-order dimensions of generalized distress; anxious arousal, or somatic anxiety; and anhedonia and positive affect (
14,
15). Items were initially selected on the basis of previous conceptual and empirical work on the dimensionality of emotion and the structure of psychopathology (
19,
29).
Final item selection and initial validation studies were conducted among both healthy samples and groups of patients who were experiencing anxiety, depressive disorders, and other disorders. Selected items were then subjected to factor analyses that yielded these three dimensions, supporting the tripartite model (
14). Items within a dimension tend to be highly correlated, whereas the correlation between dimensions is relatively low.
Examination of the items in the MASQ helps provide a more concrete sense of the symptoms and mood states that are indicators of the dimensions of general distress, anhedonia or low positive affect, and somatic anxiety.
Table 1 lists selected items from this instrument that load on these three higher-order dimensions of emotion. Three MASQ subscales relate to the higher-order dimension of generalized distress: anxiety, depression, and mixed. These scales assess affective states or symptoms that are typically associated with anxiety, depression, or a mixed anxious-depressed profile, respectively.
However, these subscales are highly correlated with one another—for example, r=.78 for the anxiety and depression subscales in a sample of 470 patients (
14)—and items from all three subscales load on the broader dimension of generalized distress. Thus all three subscales tend to reflect features that are shared by anxious and depressed patients more than elements that distinguish depressive and anxiety disorders from each other. Indeed, it is likely that many manifestations of psychopathology are associated with elevated scores for indicators of generalized distress.
In contrast, scales that tap the dimensions of anhedonia and somatic anxiety have better discriminant validity. Relative to the generalized distress dimension, the anhedonia dimension tends to be much more specific to depression. That is, the correlation between anhedonia and measures of depression tend to be notably higher than correlations between anhedonia and measures of anxiety (
14). The anhedonia dimension reflects the mood dimension of motivation, energy, and pleasure that reflects an active engagement with—as opposed to withdrawal from—rewarding stimuli from the external environment. We can readily see that the inhibition of appetitive emotions and behaviors is more specific to depressive disorders.
In contrast, anxious arousal, which primarily assesses somatic components of anxiety, as shown in
Table 1, shows notably higher correlations with measures of anxiety, particularly panic, than with measures of depression (
14). The anxious arousal subscale maps most closely onto the dimension of acute fear response to specific environment cues that we have described. Consistent with these observations are the correlations between specific MASQ scales observed by Watson and colleagues (
14). Across five samples of patients and healthy subjects, the average correlation between the anxiety and depression subscales of the generalized distress dimension was .69, whereas the average correlation between subscales of the anxious arousal and anhedonia dimensions was only .34. Thus the subscales of the latter two dimensions have superior discriminant validity. This observation also supports the contention that the distress dimension is common to depressive and anxiety disorders.
Several empirical studies have tested this tripartite model of mood among patients with depressive and anxiety disorders. Although some unanticipated complexities have emerged, in general the results have been supportive of the model.
For example, Brown and colleagues (
16) performed a factor analysis of the self-report responses of a large group of patients who had depressive and anxiety disorders. They found that the higher-order dimension of generalized distress was associated with all anxiety and depressive disorders. Somatic anxiety, on the other hand, was relatively specific to panic disorder. The positive emotional factor loaded inversely on both depression and social phobia, suggesting that anhedonia or low positive affect is linked to symptoms associated with depression but also with the social withdrawal associated with social phobia. The results of several additional studies also supported the tripartite formulation of Watson and Clark and colleagues (
20).
In several respects the tripartite model has heuristic value for researchers and clinicians alike. First, this model helps account for the symptoms that are common to anxiety and depressive disorders. High levels of distress can be seen to reflect a common set of features of anxiety and depression, whereas anhedonia and somatic anxiety reflect unique aspects of depression and panic disorders, respectively. The notion that symptoms of distress represent shared or overlapping features of anxiety and depression is consistent with the view that these symptoms are dimensional rather than categorical elements of psychopathology (
14,
30).
Second, the tripartite model has important implications for clinicians and researchers who are interested in improving treatment outcomes. If, in fact, the generalized distress and anhedonia dimensions of depression are separable, changes in one dimension need not be a strong predictor of changes in the other. Thus a depressed person could experience a decline in negative affect without a corresponding increase in positive affect. These points underscore the importance of separately and comprehensively assessing these dimensions of depression and of applying interventions aimed at specific dimensions.
One reason that measures such as the MASQ may prove to be important in the assessment of treatment outcomes is that typical rating measures for depressive and anxiety disorders tend to be highly correlated with each other (
15). Persons with either anxiety or depressive disorders tend to have elevated ratings on symptomatic measures of both anxiety and depression, such as the Ham-D and the Hamilton Rating Scale for Anxiety. This may be because many anxiety and depression rating scales are heavily weighted toward symptoms of general distress and cannot discriminate between dimensions of mood. Therefore, evidence of symptomatic improvement that is based on these instruments may primarily reflect a reduction in the symptoms of general distress that are common to both anxiety and depressive disorders.
Mood dimensions and response to antidepressants
The next question is whether an understanding of these mood dimensions is particularly helpful in making decisions about treatment. Here we review information on the relationship between the neurochemistry that is relevant to the actions of antidepressant drugs and dimensions of mood. In both animal and human models of psychopathology, symptoms of general distress and somatic anxiety appear to depend to a significant extent on the serotonin (5-HT) system (
31,
32). Drugs such as the 5-HT
1B/2A/2C receptor agonist methachlorophenylpiperazine (
33) and the 5-HT
1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tertralin (
34) have been shown to be anxiogenic in studies of both humans and animals, suggesting an important role for these receptors in the genesis of anxiety.
Furthermore, SSRIs and serotonin receptor modulators—for example, mirtazapine and nefazodone—reduce symptoms of anxiety and panic disorders (
35) in humans and inhibit behaviors that are linked to anxiety in animals (
32). Together, these findings support the notion of serotonin modulation of the distress and somatic anxiety domains. Consistent with speculations by Petty and colleagues (
30), such modulation may reflect the fact that serotonin promotes the broader maintenance of emotional and behavioral homeostasis in the face of stress.
Evidence suggests that antidepressants that act mainly through serotonin modulate primarily the general distress dimension (
36). For example, Knutson and associates (
37) administered either paroxetine or placebo to healthy volunteers in a double-blind manner over the course of four weeks. SSRIs were associated with a decrease in distress but no significant changes in positive affect. Clinical studies of the specific symptoms affected by SSRIs also are consistent. Bodkin and colleagues (
38) found that SSRIs significantly reduced symptoms of panic and anxiety in 18 of 20 patients with depression. However, these patients did not report increased energy. Indeed, ten of 21 patients reported decreased energy during treatment with SSRIs.
This observation contrasts with the effects of tricyclic antidepressants, which, as a rule, act via combined serotonergic and noradrenergic mechanisms and tend to produce relatively rapid effects on a broad set of symptoms, including symptoms associated with distress and appetitive motivation (
39). When considered from the perspective of the tripartite model, these results suggest that SSRIs reduce levels of general distress and anxious arousal but have limited effects on the anhedonia dimension, perhaps even worsening these symptoms somewhat. Such a conclusion underscores the importance of assessing the effects of medications on specific components of depression.
As a cautionary note, we should add that although there appear to be strong links between serotonergic functioning and the inhibition of generalized distress and negative affect, the specificity of these links is unclear. As several commentators have noted (
40,
41), an alternative view is that serotonin has general inhibitory functions that modulate both positive and negative affect. Consistent with this view, Zald and Depue (
41) recently found that among healthy volunteers, the maximum prolactin response to d,l-fenfluramine (a serotonin agonist), a measure of the responsiveness of the serotonin system, was inversely correlated with ratings of both daily negative (r=-.42) and positive (r=-.47) emotions. These findings are consistent with a view that the role of serotonin could be understood in a broader context of emotional regulation. Such regulation has been referred to as emotional constraint—that is, the inhibition of both positive and negative affect as well as other cognitive and affective processes (
40). Such findings introduce some ambiguity about the precise effects of SSRIs on symptoms of depression.
However, the two alternative views of the actions of serotonin or of drugs such as SSRIs—that they may have a relatively selective inhibitory effect on negative affect as opposed to inhibition of both positive and negative emotion—suggest that an SSRI would not be the medication of choice if a primary goal was the reversal of anhedonia and an increase in positive affect. In fact, the inhibition of both positive and negative emotions by serotonin could explain the "flatness" of mood that some patients experience while taking SSRIs.
A variety of basic studies have implicated noradrenergic mechanisms in the genesis of anxiety (
42,
43,
44,
45,
46). Noradrenergic agents have been shown to reduce symptoms of panic as well (
47,
48), possibly through their effects on locus coeruleus activity (
45). However, a study by Pohl and associates (
49) showed that although the noradrenergic antidepressant desipramine has antipanic properties, it was associated with selective increases in the anxiety-related side effect of jitteriness among patients with panic disorder. Also, the antianxiety effects of imipramine in generalized anxiety disorder were found to be inversely proportional to the concentrations of the desipramine metabolite (
50). Thus the noradrenergic agents may produce a specific benefit among patients with panic disorder while somewhat worsening the symptoms of anxiety overall in acute treatment.
Alternatively, several sources suggest that the dimension of positive affect is much more dependent on catecholaminergic activity, particularly dopaminergic activity (
51). Depue and colleagues (
52) showed that positive emotion was associated with dopaminergic activity, specifically a decrease in prolactin and an increase in spontaneous blink rates in response to the dopamine receptor agonist bromocriptine. There is evidence that both changes reflect functional dopaminergic activity, perhaps as a result of linkages between the trait of positive emotionality and postsynaptic dopamine receptor sensitivity. This finding, in turn, is broadly consistent with a large body of literature that links dopamine and brain reward systems.
However, as Salamone and colleagues have suggested (
11), dopaminergic activity may not mediate reward per se but, rather, the capacity to engage in effortful behaviors to attain reward. For example, dopamine D
2 receptor antagonist drugs such as pimozide and haloperidol do not reduce reward when the "cost" of acquisition is low—that is, when there are no obstacles to be surmounted. However, reduction of dopaminergic activity—for example, in the nucleus accumbens—reduces the willingness of rats to overcome restraints in obtaining a food reinforcement. Thus dopamine appears to be associated more closely with effortful behaviors that reflect heightened appetitive motivation than with the pleasure or consummatory response to rewards per se (
11).
On the basis of the evidence we have presented, it might be expected that medications or other biological manipulations that affect dopaminergic systems would result in a more pronounced effect on anhedonia. Collectively, these data suggest that serotonin may be more important for the modulation of fear responses, such as general distress, whereas dopamine may be more closely associated with anhedonia or, more specifically, low motivation.
Mechanisms of action of antidepressants
The mechanisms of action of currently available antidepressants include the enhancement of transmission of monoamines, such as serotonin, norepinephrine, and dopamine, and the antagonism of serotonin receptors (
53). The former class includes reuptake blockers, such as tricyclics and SSRIs; monoamine oxidase inhibitors (MAOIs); and the alpha
2 antagonist mirtazapine, which induces the release of both norepinephrine and serotonin. The latter class includes 5-HT
2 antagonists, such as nefazodone and trazodone, as well as mirtazapine, which also blocks 5-HT
3 receptors. Therefore, the proximal mechanism of action of all currently available antidepressants involves the regulation of synaptic transmission via the monoamines.
Many drugs are relatively selective for one of the monoaminergic systems—for example, serotonin for the SSRIs and norepinephrine for reboxetine and desipramine. However, before we assume that these agents are truly selective, it is important to keep in mind that there is considerable co-localization and "cross-talk" of monoamines in the central nervous system (
54). That is, monoamines are mutually regulating. Norepinephrine itself as well as norepinephrine selective reuptake inhibitors (NSRIs) enhance the release of both dopamine and serotonin in the forebrain (
55).
The effect of NSRIs on serotonin and dopamine may be explained by the observation that norepinephrine, acting through alpha
1 receptors, can induce release of these transmitters (
54). However, in addition, the norepinephrine transporter protein—the reuptake site—also has high affinity for the reuptake of dopamine (
56). Thus NSRIs are, in effect, dopamine reuptake inhibitors as well. This concept is supported by Karson's finding (
57) that spontaneous blink rates—as noted, a putative measure of forebrain dopamine activity—were elevated among depressed patients who were taking tricyclic antidepressants. Thus enhancement of norepinephrine transmission—by reuptake blockade or by a reduction in the metabolism of norepinephrine by inhibition of monoamine oxidase—would be expected to induce an enhancement of arousal via the norepinephrine mechanisms (
58) but also to enhance reward acquisition—that is, motivation—through actions on dopaminergic systems.
The interplay of serotonin with norepinephrine and dopamine is more complicated because of the complexity of the serotonin receptor system relative to the catecholamines. For example, serotonin has been shown to both enhance and inhibit dopaminergic activity in the frontal cortex and nucleus accumbens, depending on the type of serotonin receptor subtype that is activated (
21). The inhibition of dopamine by serotonin—primarily via 5-HT
2C receptors (
59,
60,
61)—may explain why highly selective SSRIs have been shown to reduce dopamine release in the frontal cortex or nucleus accumbens (
62,
63) and reward acquisition in animals (
64,
65,
66).
This effect may parallel the mood-flattening effect of these agents among some patients and is consistent with the concept of a link between serotonin and constraint of dopaminergic systems as posited by Depue and colleagues (
40). Clinically, it is important to keep in mind that not all patients who are treated with SSRIs will experience a flattening effect. However, it has been our observation that when a therapeutic failure occurs with one of these agents, it is often the result of a lack of improvement in the anhedonia experienced by many depressed patients.
A therapeutic heuristic
Clearly depression is a serious illness that is associated with a level of functional impairment as great as or greater than that associated with other medical conditions (
7,
67). Further, as we have noted, a simple amelioration in symptoms may not lead to full functional recovery (
7). We have argued that it is possible to separate depressive symptoms into two relatively independent constructs: general distress and anhedonia. In fact, we suggest that separating depressive symptoms into those most closely akin to anxiety—fear, negative rumination, and anxious mood—and those indicative of low pleasure and motivation can provide a framework for evaluating the adequacy of response and determining the next step in treatment.
As we have noted, depression rating scales tend to be heavily weighted toward the distress dimension of mood (
15) at the expense of the positive affective dimension. Rating instruments such as the Social Adjustment Scale (SAS) (
67) and the Social Adaptation Self-Evaluation Scale (SASE) (
68) may be useful to some clinicians in evaluating residual impairment in important areas of role functioning, such as work, housework, and parenting, which could in turn be considered to be related to the positive emotion-high motivation dimension.
As an alternative to these relatively complicated rating instruments, Stahl (
69) recently provided a simple clinical method for assessing functional outcome: asking "What are three signal events in your life that you do when you are well but not when you are depressed or anxious?" The assessment of these events over time may provide a simple but effective assessment of functional outcome linked to motivation.
A careful assessment of both symptomatic and functional outcomes often leads to the realization that the patient will experience an incomplete outcome with any given antidepressant treatment. The question then becomes "What next?" Here, the "functional assay"—that is, the question "Has your functioning returned to normal?"—takes us to the stage of deciding on the next strategy in therapy.
Response rates—that is, the likelihood that a patient will experience significant positive change from baseline—do not differ much between antidepressants. However, remission—or complete recovery—does appear to vary between drugs. In particular, tricyclic antidepressants appear to produce remission rates that are higher than those achieved with SSRIs among patients who are more severely depressed, especially those with melancholia (
70,
71,
72). Furthermore, it has been suggested that venlafaxine may be associated with a small but significantly greater remission rate than that associated with the SSRIs (
73).
In a recent pooled analysis of all clinical trials that compared venlafaxine with SSRIs—fluoxetine, paroxetine, and fluvoxamine—Thase and colleagues (
73) found a remission rate of 45 percent for patients treated with venlafaxine, compared with 35 percent for patients who received SSRIs and 25 percent for patients who received placebo. This difference between drugs has been attributed to the combined effects on serotonin and norepinephrine (
69), although this point remains controversial (
74). Given the data we have reviewed here, combining serotonergic and noradrenergic mechanisms might be expected to broaden the effects of the antidepressants by targeting both symptoms linked to the enhancement of pleasure and pleasurable engagement with the environment and symptoms linked to the experience of distress.
In support of this view is the finding that combining a noradrenergic antidepressant, such as desipramine (
75) or bupropion (
76,
77), with an SSRI enhances the antidepressive effect among persons who do not respond to either SSRIs or NSRIs. This certainly suggests that some depressed patients who do not respond to selective agents taken alone will benefit from a combination of serotonin and norepinephrine. This suggests the possibility of a broader effect on both distress and positive motivational symptoms.
Do some patients fare better with SSRIs? One possible answer is that patients who have depression with atypical features achieve better outcomes. This depressive subtype is characterized by reversed vegetative features, including hypersomnia, hyperphagia, dense fatigue, and weight gain. However, other prominent features of atypical depression include anxiety and positive mood reactivity—that is, a tendency for mood to rebound briefly after a positive event (
78,
79,
80). Atypical depression has been shown to respond more positively to MAOIs than to tricyclic antidepressants (
78). More recent data suggest that SSRIs also are superior to tricyclic antidepressants (
81) and equivalent to MAOIs (
82). Alternatively, a recent large-scale trial found both imipramine and fluoxetine to be superior to placebo and equivalent to each other (
83). As we have noted, desipramine, the more purely noradrenergic metabolite of imipramine, actually counteracted the antianxiety effects of imipramine among patients with anxiety (
50). This observation suggests that the beneficial effects of imipramine on anxiety symptoms are mediated serotonergically (
50).
In support of the serotonin distress mechanism for atypical depression, McGrath and colleagues (
84) showed that gepirone, a 5-HT
1A partial agonist agent similar to the antianxiety drug buspirone, was much more effective than placebo in a group of patients with atypical depression; the response rates were 62 percent and 20 percent, respectively. The collective data suggest that serotonergic antidepressants, including SSRIs, MAOIs, and perhaps imipramine and gepirone, have benefit in atypical depression.
Overall these data suggest that melancholia, typified by persistent anhedonia and positive vegetative features, may respond best to noradrenergic—or mixed noradrenergic and serotonergic—agents. Alternatively, atypical depression, with prominent anxiety and reactive mood, appears to respond well to serotonergic drugs. These findings will help lead us to a framework for managing depression.
In dealing with treatment-resistant depression, the key question is "What is the next step?" There are many possible choices, including use of alternative monotherapies and augmentation. These options were recently reviewed extensively (
85,
86,
87,
88). When depression has not yet resolved, what symptoms remain? Although many patients will have residual distress and anhedonia, often one feature or the other will predominate.
Liebowitz (
89) has suggested that depression with prominent anxiety or atypical features responds well to serotonergic agents such as MAOIs and SSRIs. As we have noted, serotonergic treatments may be somewhat more important for symptoms of distress and may, under certain circumstances, actually worsen symptoms in the domains of pleasure and motivation. Alternatively, consistent with the effects of tricyclic antidepressants in melancholia, the catecholamines (norepinephrine and dopamine) may be more significant.
Although we cannot draw an absolute dichotomy here, these observations may provide a framework for making decisions about treatment. The heuristic, then, could be that if distress is the predominant residual symptom, pharmacological approaches that affect the serotonin system might be the best initial step. Alternatively, if the lack of appetitive motivation is the main problem, treatments that focus on the catecholamines might be chosen, as illustrated in
Table 2. Even though dopamine may be more significant for appetitive motivation, the interplay between norepinephrine and dopaminergic systems suggests that noradrenergic agents will indirectly produce effects on functional dopamine activity.
As noted, many antidepressants act via serotonin-dependent mechanisms, and choosing an antidepressant with serotonin reuptake blocking properties or serotonin receptor antagonist properties can be expected to reduce symptoms of distress (
53). However, augmentation strategies that emphasize actions in the serotonin system also may be useful when distress symptoms predominate in the clinical picture. Therefore, a switch to a more potent serotonergic agent or the use of augmenting agents such as lithium (
90,
91,
92) and buspirone (
93) that have known serotonergic effects may be the preferred initial choices for treating a highly distressed patient.
Many patients experience residual problems with energy and appetitive motivation. For these patients, the model suggests using a catecholaminergic antidepressant as monotherapy. However, the use of augmenting drugs that act through catecholaminergic mechanisms, such as desipramine and bupropion (
94,
95), or psychostimulant drugs, such as methylphenidate and methamphetamine (
96), in combination with a serotonergic agent also could prove useful. More recently, the atypical antipsychotics have been shown to be an effective augmenting strategy when used with SSRIs to treat refractory unipolar depression among patients who are not psychotic (
97).
For example, Ostroff and Nelson (
98) reported that the addition of risperidone to an SSRI was an effective augmentation strategy for eight treatment-resistant patients. Shelton and colleagues (
97) conducted a clinical trial of augmentation of fluoxetine with olanzapine among patients with treatment-resistant depression. The participants in that study had not responded to either NSRIs or SSRIs, and they received a prospective run-in with 40 to 60 mg of fluoxetine a day. Nonresponders were randomly assigned to receive continuation fluoxetine plus placebo, 5 to 20 mg of olanzapine a day plus placebo, or the olanzapine-fluoxetine combination. The combined treatment produced a rapid response that was sustained over a 16-week follow-up period in about half of the patients, which was not seen in the other groups.
In a related study, Zhang and associates (
99) showed that when fluoxetine in combination with olanzapine or risperidone was administered to rats, a marked elevation was observed in frontal norepinephrine and dopamine without a concomitant change in serotonin. This finding suggests that the combination treatment benefits a residual lack of energy and appetitive motivation.
It would be remiss of us to suggest that pharmacotherapies should be the primary approach to resistant depression. Psychotherapy, particularly cognitive-behavioral therapy and interpersonal psychotherapy, have been shown to be beneficial in the treatment of depression. Psychotherapy should certainly be considered as an adjunct in the treatment of patients who have not achieved full remission with antidepressants. More recently, an adaptation of the cognitive therapy model called the cognitive-behavioral analysis system of psychotherapy (
100,
101) was shown to be effective when used in combination with an antidepressant among chronically depressed patients.
Given the variety of pharmacological and psychotherapeutic options currently available, recovery seems to be an achievable goal for most patients who have depression. However, to reach this end, we must have tools that will enable us to know when the goal has not been achieved and to determine what steps to take next. We hope that the concepts in this article will contribute to a move in that direction.
It is important to keep in mind that this is a working model only and that alternative choices, such as augmentation with lithium for an anergic patient and desipramine for an anxious one, may still be useful in some circumstances. Many of the studies of mood dimensions that we have reviewed drew from populations of healthy volunteers or patients from research clinics. Thus the results may be only weakly generalizable to patients encountered in practice. Furthermore, this model does not suggest that treatment should be altered in the case of a patient who is doing well, regardless of the patient's previous symptoms. However, given our current state of knowledge, this conceptual approach may be helpful for planning initial treatment strategies. It is likely that a more complex understanding of specific phenotypes of mood disorders will emerge in the future and will help to guide selection of treatment.