Subjects
The mean Karnofsky Performance Status score for the 30 patients was 52.3 (SD=21.3, range=10–90). ANOVA showed that there were no significant differences among drug treatment groups in age, education, or Karnofsky scores.
Medical Status Profile data showed that patients had multiple medical complications (mean=12.57, SD=4.1, range=6.00–22.00). Most common were hematologic and metabolic disorders (e.g., anemia, leukopenia, thrombocytopenia, hypoalbuminemia) and infectious diseases (e.g., septicemia, systemic fungal infections, pneumocystis carinii pneumonia, tuberculosis, and disseminated viral infections). Severity of medical complications for the study group was in the moderate to severe range (mean=2.44, SD=3.1, range=1.75–2.87). There were no differences (ANOVA) among groups in number of medical complications (F=1.21, df=2, 23, p<0.32) or severity of these complications (F=0.51, df=2, 23, p<0.61).
The context and course of the delirium episode varied among patients. Multiple etiologies, rather than a single etiology, usually accounted for the delirium episode. Some deliria occurred in the context of major organ system failure or during the dying process, which limited the effectiveness of treatment. In other cases, the delirium was a temporary phenomenon in a healthier patient and was treated successfully. Five patients (two in the haloperidol group, two in the chlorpromazine group, and one in the lorazepam group) died within 8 days of initiation of the protocol. Four patients (three in the chlorpromazine group and one in the lorazepam group) died within 1 week after completing the protocol. Seventeen patients lived longer than 2 weeks following day 1 of the protocol (mean=183 days, range=19–1,305). Four patients were known to have lived for a minimum of 2–7 months (mean=70 days) following treatment, but their specific date of death was not known.
Efficacy
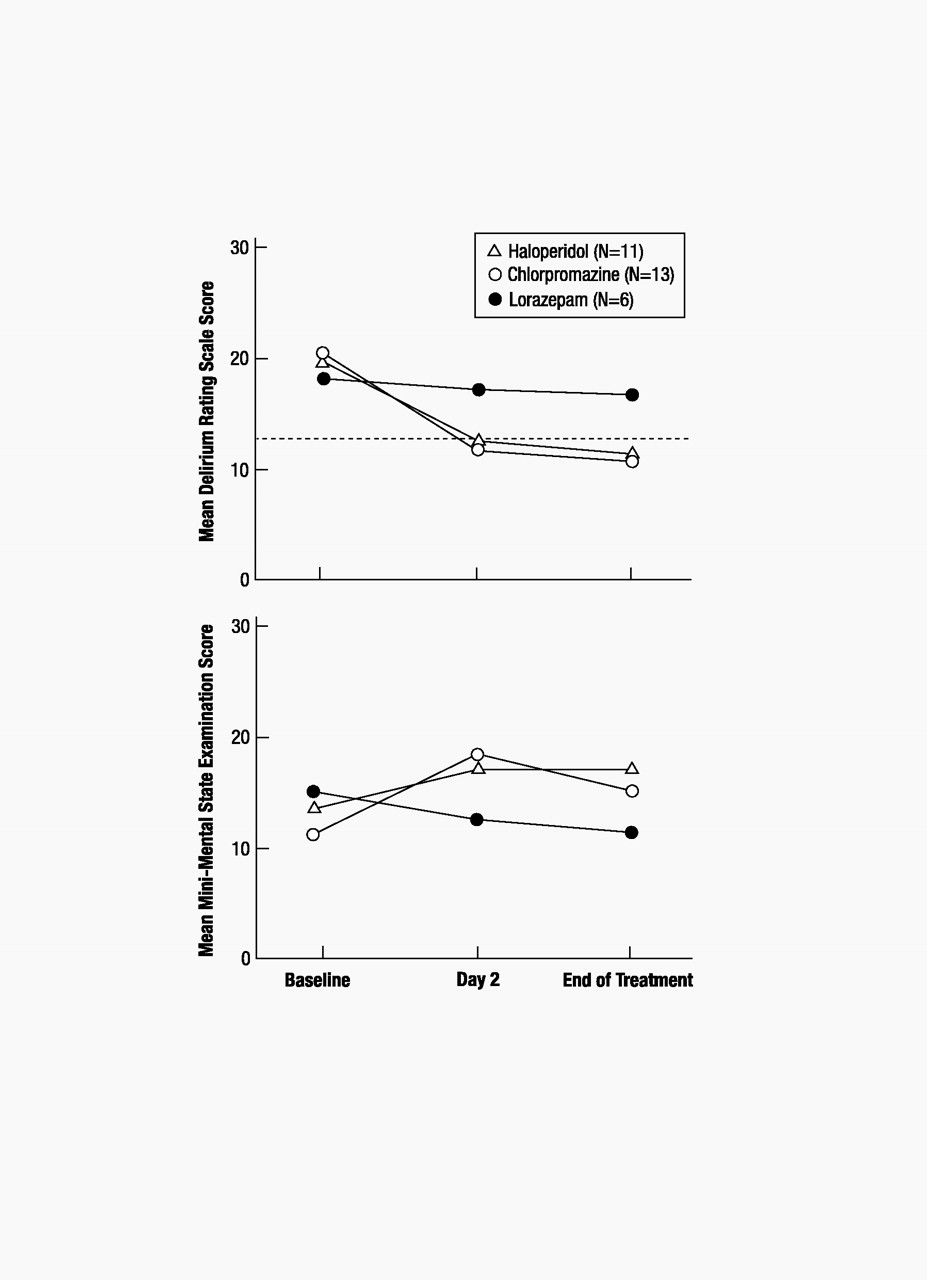
Delirium Rating Scale scores for the total group (N=30) averaged 20.1 at baseline (SD=3.5, range=14–28), 13.3 on day 2 (SD=6.1, range=3–26), and 12.8 at the end of treatment (SD=6.4, range=3–26). Mini-Mental State scores for the group averaged 12.7 (SD=7.6, range=0–26) at baseline, 16.8 (SD=9.8, range=0–30) on day 2, and 15.1 (SD=10.6, range=0–30) at the end of treatment.
Delirium symptoms, as measured by the Delirium Rating Scale, improved, to values below the diagnostic threshold for delirium, for both the haloperidol and chlorpromazine groups but not for the lorazepam group. This improvement occurred in the neuroleptic drug groups within the first 24 hours of treatment (between baseline and day 2), and there was little or no further improvement between day 2 and the end of treatment. Delirium Rating Scale scores for the haloperidol group averaged 20.45 (SD=3.45) at baseline, 12.45 (SD=5.87) on day 2, and 11.64 (SD=6.10) at the end of treatment. Delirium Rating Scale scores for the chlorpromazine group averaged 20.62 (SD=3.88) at baseline, 12.08 (SD=6.50) on day 2, and 11.85 (SD=6.74) at the end of treatment. Delirium Rating Scale scores for the lorazepam group averaged 18.33 (SD=2.58) at baseline, 17.33 (SD=4.18) on day 2, and 17.00 (SD=4.98) at the end of treatment. Analysis of changes in delirium scale scores revealed a main effect for time (F=10.09, df=2, 27, p<0.001) but not for drug (F=0.84, df=2, 27, p<0.44). Simple effects analyses were conducted on the basis of a priori hypotheses regarding between-drug differences in efficacy and were supported by the overall drug-by-time interaction, which approached significance (F=2.27, df=4, 54, p<0.07). As shown in figure 1, there was a significant decrease in Delirium Rating Scale scores from baseline to day 2 for the two neuroleptic groups but not for the lorazepam group (haloperidol: F=27.50, df=1, 27, p<0.001; chlorpromazine: F= 37.02, df=1, 27, p<0.001; lorazepam: F=0.23, df=1, 27, p<0.63). There were no significant changes for any of the three drug groups in delirium scale scores from day 2 to the end of treatment (haloperidol: F=0.63, df=1, 27, p<0.43; chlorpromazine: F=0.06, df=1, 27, p<0.81; lorazepam: F=0.06, df=1, 27, p<0.81).
Cognitive status, as measured by the Mini-Mental State, improved between baseline and day 2 for the neuroleptic groups, but that improvement was significant only for the chlorpromazine group. The lorazepam group did not show improvement. There was no further improvement between day 2 and the end of treatment for any of the drug groups. Mini-Mental State scores for the haloperidol group averaged 13.45 (SD=6.95) at baseline, 17.27 (SD=8.87) on day 2, and 17.18 (SD=12.12) at the end of treatment. Mini-Mental State scores for the chlorpromazine group averaged 10.92 (SD=8.87) at baseline, 18.31 (SD=10.61) on day 2, and 15.08 (SD=10.43) at the end of treatment. Mini-Mental State scores for the lorazepam group averaged 15.17 (SD=5.31) at baseline, 12.67 (SD=10.23) on day 2, and 11.50 (SD=8.69) at the end of treatment. Analysis of changes in Mini-Mental State scores revealed a main effect for time that approached significance (F=2.91, df=2, 26, p<0.07) but not a main effect for drug (F=0.22, df=2, 27, p<0.81). The drug-by-time interaction approached significance (F=2.22, df=4, 54, p<0.08). As shown in figure 1, scores on the Mini-Mental State improved significantly from baseline to day 2 for patients treated with chlorpromazine (F=13.99, df=1, 27, p<0.001); there was a trend toward improvement for those treated with haloperidol (F=3.16, df=1, 27, p<0.09), but there was no evidence of improvement for patients treated with lorazepam (F=0.74, df=1, 27, p<0.40). Scores on the Mini-Mental State from day 2 to end of treatment declined for patients treated with chlorpromazine (F=4.68, df=1, 27, p<0.04) and did not significantly change for patients treated with either of the other two drugs (haloperidol: F=00, df=1, 27, p<0.96; lorazepam: F=0.28, df=1, 27, p<0.60).
Early terminations/side effects.
All six patients who received lorazepam developed treatment-limiting side effects, including oversedation, disinhibition, ataxia, and increased confusion, leading to refusal to take the drug or requiring discontinuation of the drug. As noted earlier, because of the experience of these patients, lorazepam was removed from the protocol, and all remaining patients were randomly assigned to either haloperidol or chlorpromazine. No clinically significant medication-related side effects were noted in the neuroleptic drug groups during the trial period.
Extrapyramidal side effects.
None of the patients in the study developed dystonic or dyskinetic symptoms during the period of drug therapy. Extrapyramidal symptoms were noted only on the objective parkinsonism subscale of the Extrapyramidal Symptom Rating Scale, and they were limited primarily to decreased expressive automatic movements, rigidity, tremor, and mild akathisia. Scores on the parkinsonism subscale were extremely low at baseline before medication (haloperidol: mean=7.00, SD=6.80; chlorpromazine: mean=7.42, SD=8.08; lorazepam: mean=7.60, SD=10.11) and during maintenance therapy (haloperidol: mean=5.54, SD=6.76; chlorpromazine: mean=5.08, SD=4.48; lorazepam: mean=12.20, SD=8.93). Analysis of changes in Extrapyramidal Symptom Rating Scale scores (baseline to maintenance) revealed no effect for time (F=0.06, df=1, 27, p<0.81) or drug (F=0.61, df=2, 25, p<0.55) and a trend toward significance for drug-by-time interaction (F=2.98, df=2, 25, p<0.07). This interaction appeared to be due to an increase in extrapyramidal side effects in the lorazepam group. However, because of the small number of patients in the lorazepam group (N=5; one subject refused to complete the examination), caution should be used in interpreting these data; further analysis was not pursued.