There is considerable controversy regarding the rate of response to antidepressants and the risk of a switch in mood polarity into hypomania or mania when these agents are used as adjunctive treatment to mood stabilizers in bipolar illness (
1–
8). We previously reported the acute response and switch rates when one of three second-generation antidepressants (bupropion, sertraline, or venlafaxine) was added to mood stabilizers in a 10-week randomized acute treatment trial (
9).
Here we present 1) results of a more extended evaluation of the same patient cohort in which we used a continuous daily mood measure (the National Institute of Mental Health [NIMH] Life Chart Method [LCM] [10, 11]) and 2) results from a continuation phase lasting up to 1 year for patients who responded to acute treatment. Use of clinicians’ continuous daily prospective ratings with the LCM allowed us to assess the severity and duration of four categories of hypomania or mania ranging from brief subthreshold hypomanias to a full switch in mood polarity to mania that was associated with at least some days of moderate dysfunction. We also blindly rated the degree of anti-depressant response by using the Clinical Global Impression Bipolar Version (CGI-BP) during acute and continuation antidepressant treatment.
Much of the controversy in the literature about the rates of switches in mood polarity in patients with bipolar disorder who receive antidepressants may be due to several study characteristics that are specifically addressed in this analysis. In this study, we 1) rated a continuum of mood states ranging from brief hypomania to mania with dysfunction, rather than using only a single-threshold or dichotomous measure of mood switches; 2) conducted prospective blind assessments both in the 10-week acute randomized trial phase and in the continuation phase that included putative responders to acute antidepressant treatment; and 3) included substantial numbers of patients with rapid cycling and patients without rapid cycling and of bipolar I and bipolar II disorder patients.
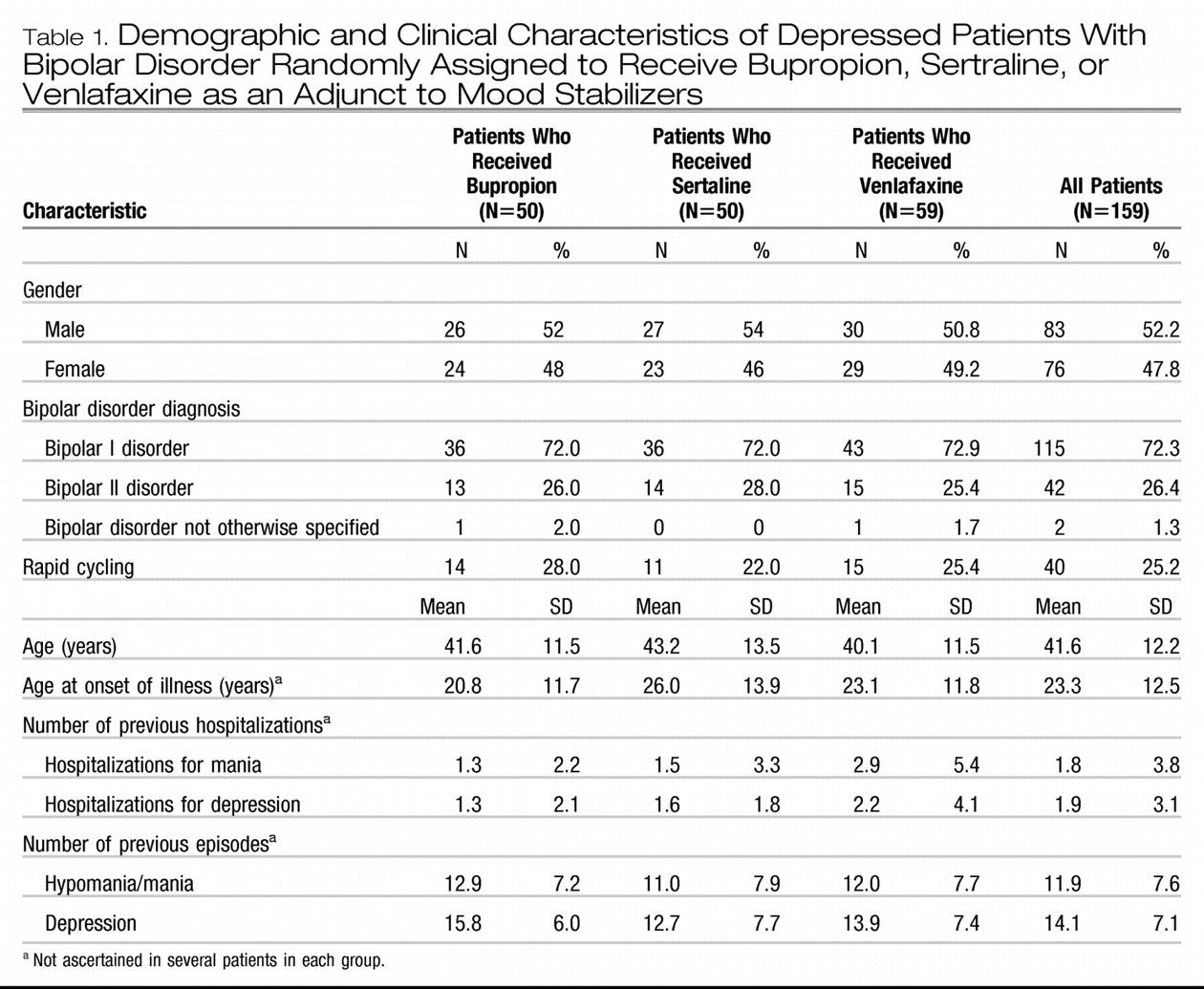
Method
The general characteristics of the patient population and the study methods have been presented in detail elsewhere (
12,
13). Briefly, all patients participated in the former Stanley Foundation Bipolar Network (
14–
16) and gave specific written informed consents for participation in both the Network and later in this specific study after the study procedures had been fully explained. The patients’ characteristics are summarized in Table 1. A total of 72.3% (N=115) of the patients received a diagnosis of bipolar I disorder according to the Structured Clinical Interview for DSM-IV Axis I Disorders, and 25.2% (N=40) of the whole cohort had rapid cycling at Network entry.
Bipolar disorder patients with depression that occurred in the context of ongoing treatment with at least one mood stabilizer at clinically therapeutic blood levels were randomly assigned to receive bupropion, sertraline, or venlafaxine adjunctively. Life charts were available for 159 of the original 184 patients described in the cross-sectional evaluation of the acute clinical trial (
9). Patients who did not respond acutely to the initial antidepressant were offered blind rerandomization to one of the other two drugs. In this fashion, 16 patients received bupropion in the second or third acute randomization, 26 received sertraline, and 27 received venlafaxine. The total number of drug exposures was 228, including 66 exposures to bupropion, 76 to sertraline, and 86 to venlafaxine. For simplicity, hereafter we will use the term “trial” to refer to each drug “exposure.” A total of 87 antidepressant continuation trials—24 for bupropion, 32 for sertraline, and 31 for venlafaxine—were assessed.
The rerandomization enabled us to assess the maximal number of acute and continuation trials for each drug. The number of continuation trials was of particular interest because we postulated that response rates would not differ among the three drugs in the acute treatment phase but that venlafaxine would have the highest switch rate in continuation treatment. This hypothesis was based on the observations of Sachs et al. (
17,
18) of a higher switch rate with the noradrenergic tricyclic agent desipramine than with bupropion.
The average maximum doses achieved in the acute phase were 286 mg/day (SD=132) for bupropion, 192 mg/day (SD=104) for sertraline, and 195 mg/day (SD=112) for venlafaxine. These doses were maintained in the continuation phase. The baseline regimen of an average of 1.96 (SD=1.01) of mood stabilizers (in the first randomization) was held constant; these drugs included lithium, anticonvulsants, antipsychotics, benzodiazepines, and ongoing thyroid hormone treatment. Adherence to the antidepressant treatment was assessed by pill counts. Study medication for the following week(s) was dispensed during study visits, and patients were required to return unused pills. Patients were seen weekly for the first 2 weeks, then at least biweekly during the 10-week acute treatment phase, and at least once every month during the continuation phase unless more frequent visits were required because of clinical status. The overall rate of adherence was high, and no significant differences were found in the pill counts among the three drugs.
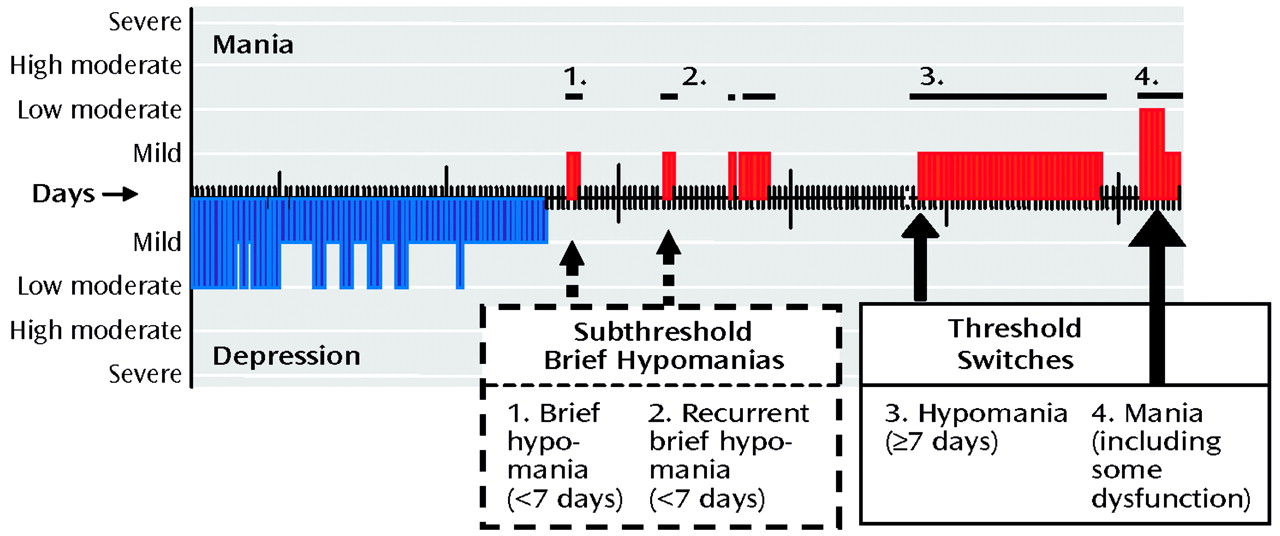
On the basis of patients’ daily reports of their degree of mood-associated dysfunction, clinicians completed the LCM daily ratings of depression or mania on a 4-point severity scale (
13,
14) during each study visit. The reliability and validity of this scale have been previously documented (
19,
20). Two raters (G.S.L. and R.M.P.), who were blind to the antidepressants used by the patients, evaluated a graphic depiction of the daily LCM ratings for each acute and continuation trial for antidepressant response and emergence of hypomania or mania (Figure 1). A CGI-BP rating of 1 (very much improved) or 2 (much improved) in depression was considered an antidepressant response.
We modified Angst’s classification (
21) to develop four categories of hypomania and mania: 1) brief hypomania, a period of at least 1 but <7 continuous days during which the patient had an LCM rating of mild severity of hypomania; 2) recurrent brief hypomania, which consisted of two or more episodes of brief hypomania over the course of a drug trial; 3) switch to full-duration hypomania, a period of ≥7 continuous days during which the patient had an LCM rating of mild severity of mania; and 4) a switch to mania, an episode that contained at least 2 days during which the patient had an LCM rating of moderate or greater severity of mania. It is noteworthy that our criterion for full-duration hypomania of ≥7 days is more conservative than the DSM-IV criteria for hypomania, which require only 4 days. The ≥7-day duration was needed to clearly distinguish full-duration hypomanias from the brief hypomanias described by Angst (
21), which have a median duration of 3 days.
For each antidepressant trial, only the most severe form of classification of hypomania/mania observed was considered in the switch rates reported here. Moreover, because the brief hypomanias would presumably have been missed or ignored in most other studies and might be considered clinically nonproblematic because of their brevity and lack of association with functional impairment, we did not consider them as full or threshold switches.
The interrater reliability for the LCM-based rating of antidepressant response and the four classifications of hypomania or mania was high, with the two blind raters independently arriving at identical categorizations 93% of the time. Minor discrepancies were readily resolved by consensus. After all ratings were completed, the blind was broken, and the data for all the acute and continuation trials as a group were analyzed first and then the data for each of the three drugs were analyzed separately. Response and switch rates were analyzed by using chi-square tests, and the ratios of brief hypomanias to full switches in the acute and continuation trials were examined. Data for trials in rapid-cycling patients were compared to those for trials in patients with non-rapid-cycling disorder. Survival analyses were performed to assess the number of days to a switch to full-duration hypomania or mania in patients with bipolar I disorder, compared to bipolar II disorder patients, and in the first randomization sequence, compared with the subsequent ones.
Results
Overall Response to Adjunctive Antidepressants and Switch Rates
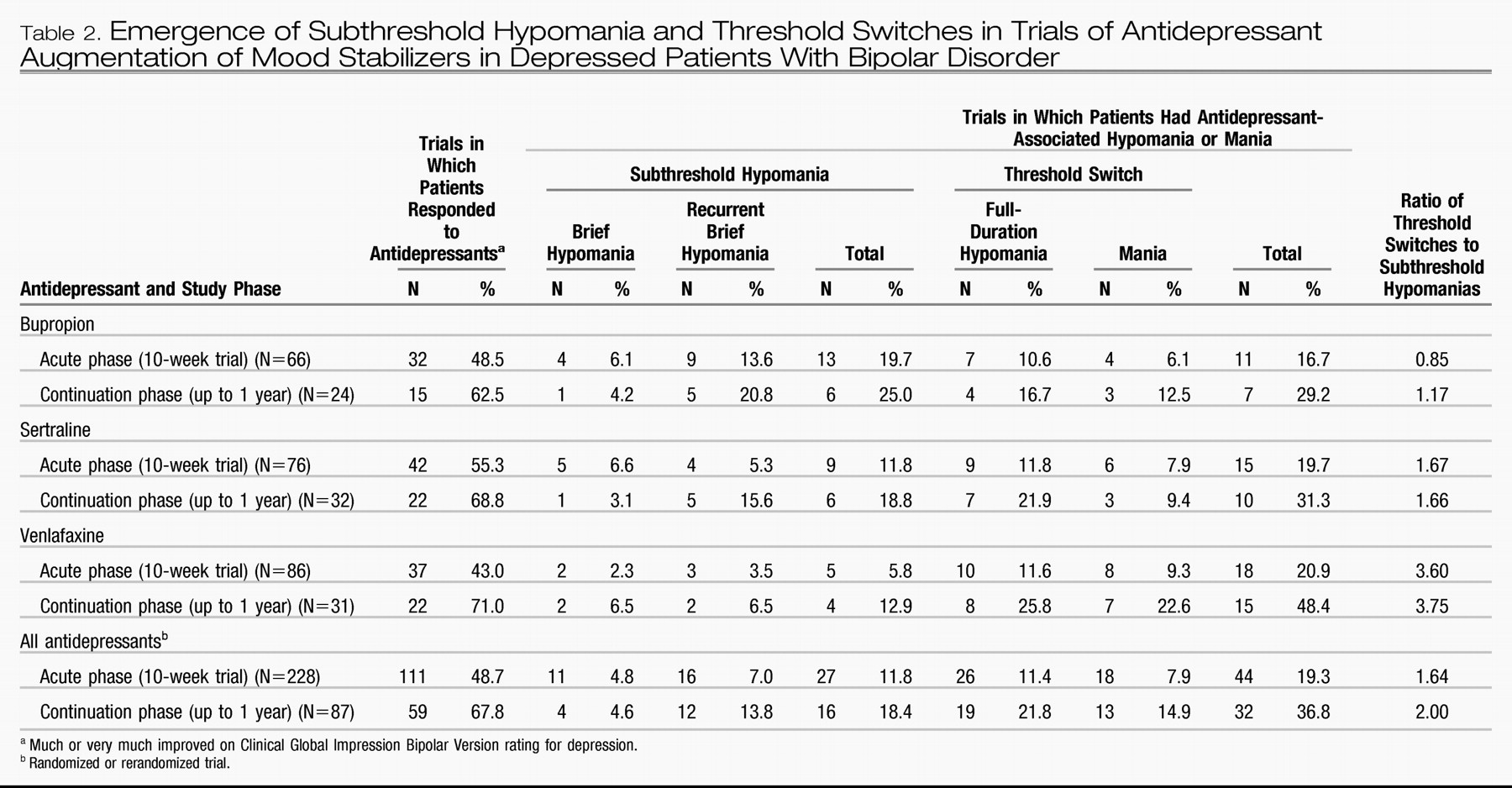
As Table 2 shows, 111 (48.7%) of the 228 acute antidepressant trials were associated with a rating of much improved or very much improved on the CGI-BP. In the acute treatment phase, brief hypomania occurred in 4.8% of the trials and recurrent brief hypomania in 7.0% of the trials. A switch into full-duration hypomania occurred in 11.4% of trials, and a switch into mania in 7.9% of trials, for a combined switch rate of 19.3% during the 10-week acute antidepressant augmentation trials. This rate is similar to the 20.7% switch rate observed in the first acute randomization by using only Young Mania Rating Scale or CGI-BP criteria (
9). In the current study, if the antidepressant responders who had a full switch (N=37) were excluded, the overall response rate in the absence of a switch was 32.5% (74 of the 228 trials). Overall, 87 (38.2%) of the 228 acute trials resulted in sufficient improvement for patients to be offered inclusion in the antidepressant continuation phase, which lasted up to 1 year. The patients with sufficient improvement included several with minimal improvement (classified here as nonresponders) and several who had a switch (but who subsequently restabilized).
As Table 2 shows, 21.8% of antidepressant continuation trials were associated with a full hypomania and 14.9% with a mania, for a combined switch rate of 36.8%. In 67.8% of the continuation trials, patients showed an antidepressant response, but 22 of the 59 antidepressant responders also experienced a switch. The response rate in the absence of a switch was 42.5% (37 of 87 evaluable continuation trials) or only 16.2% (37 of 228) of the original intentto-treat acute trials. This result rate equated to 37 of 159 patients (23.3%) who experienced a sustained antidepressant response without a switch in the continuation phase.
Survival analysis showed that the overall switch rate and the individual-drug switch rates observed in the first randomization did not differ from those in the rerandomizations. The rates of switching also were not related to the type or number of mood stabilizers received in the baseline regimen (lithium versus an anticonvulsant versus an atypical antipsychotic).
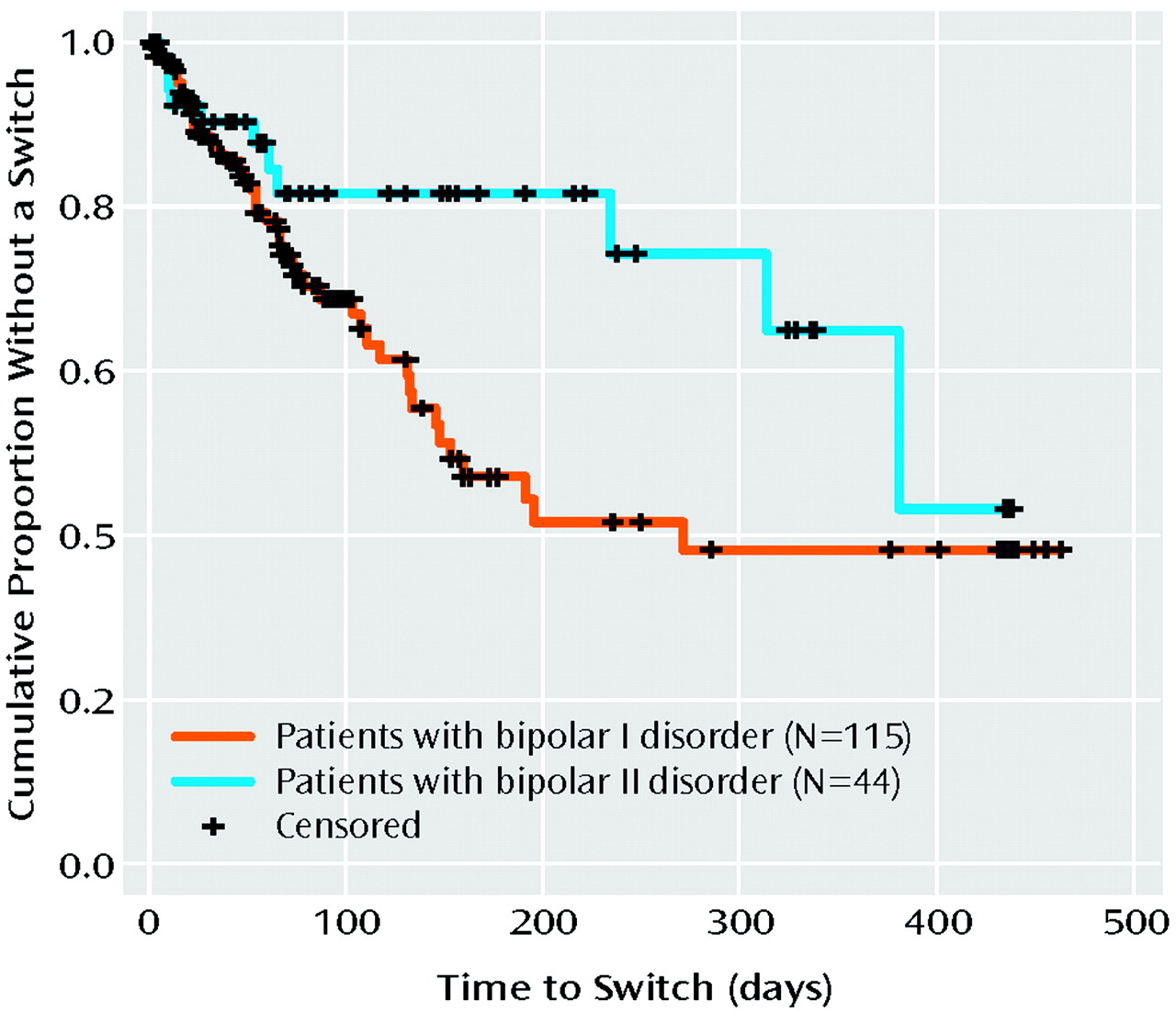
Survival analysis showed a higher rate of full switches in the trials in patients with bipolar I disorder, compared to those in patients with bipolar II disorder (Figure 2) (p=0.03). Other demographic and illness variables, including gender, age of onset, history of rapid cycling, or a past history of an antidepressant-related switch into hypomania or mania, were not significant correlates of the risk of switching in this study. A surprising result was that the patients without a positive family history of unipolar or bipolar affective illness in first-degree relatives had a higher risk of a switch during treatment with antidepressants than those who had such a family history.
Response and Switch Rates Associated With Individual Antidepressants
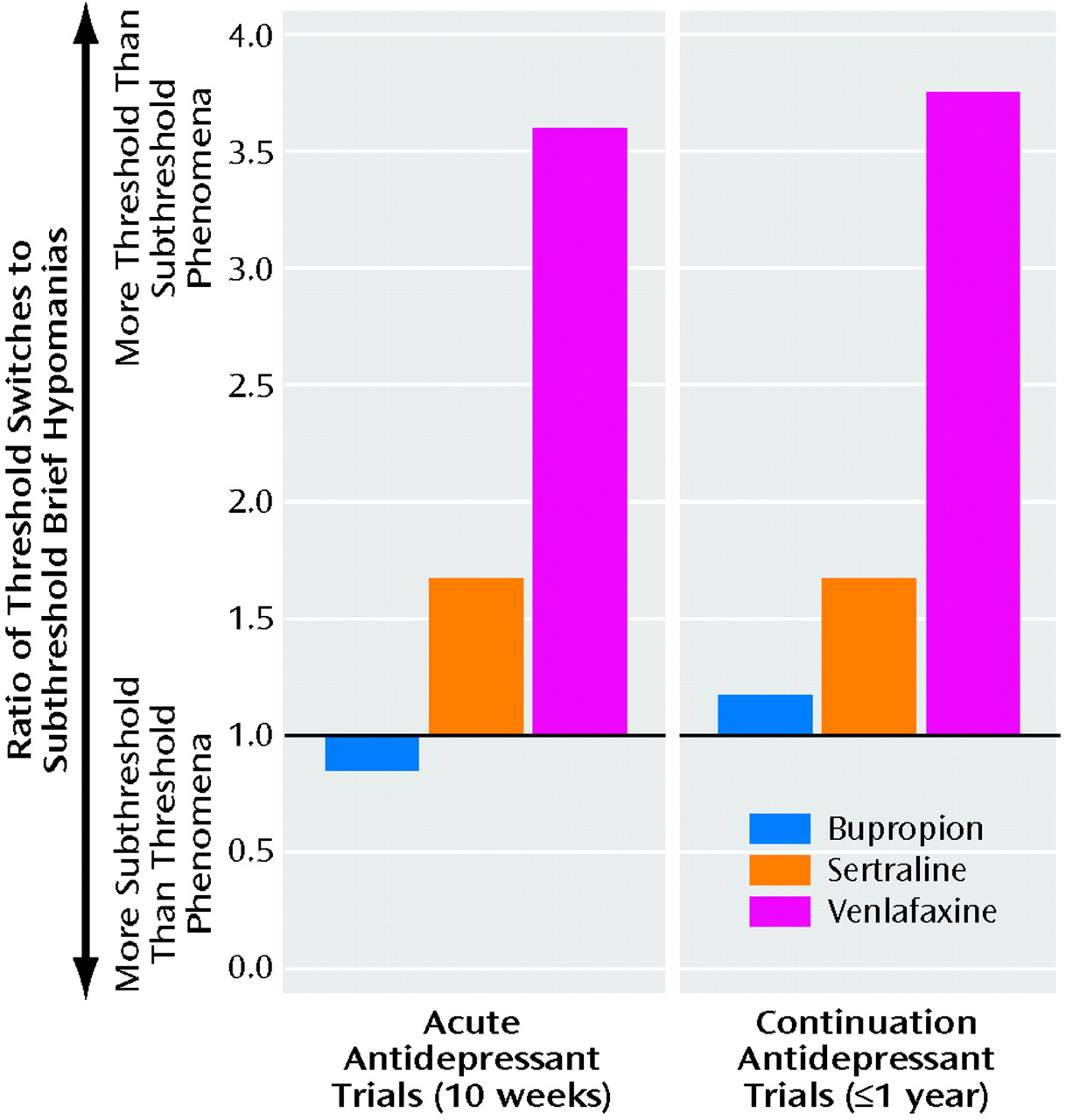
Antidepressant response and switch rates were not significantly different among the three antidepressants in either the acute or the continuation phase (Table 2). However, as Figure 3 illustrates, in both the acute and continuation phases, the ratio of threshold switches to subthreshold hypomanias was lowest with bupropion and was three times higher for venlafaxine. The ratio for sertraline was closer to the ratio for bupropion than to the ratio for venlafaxine.
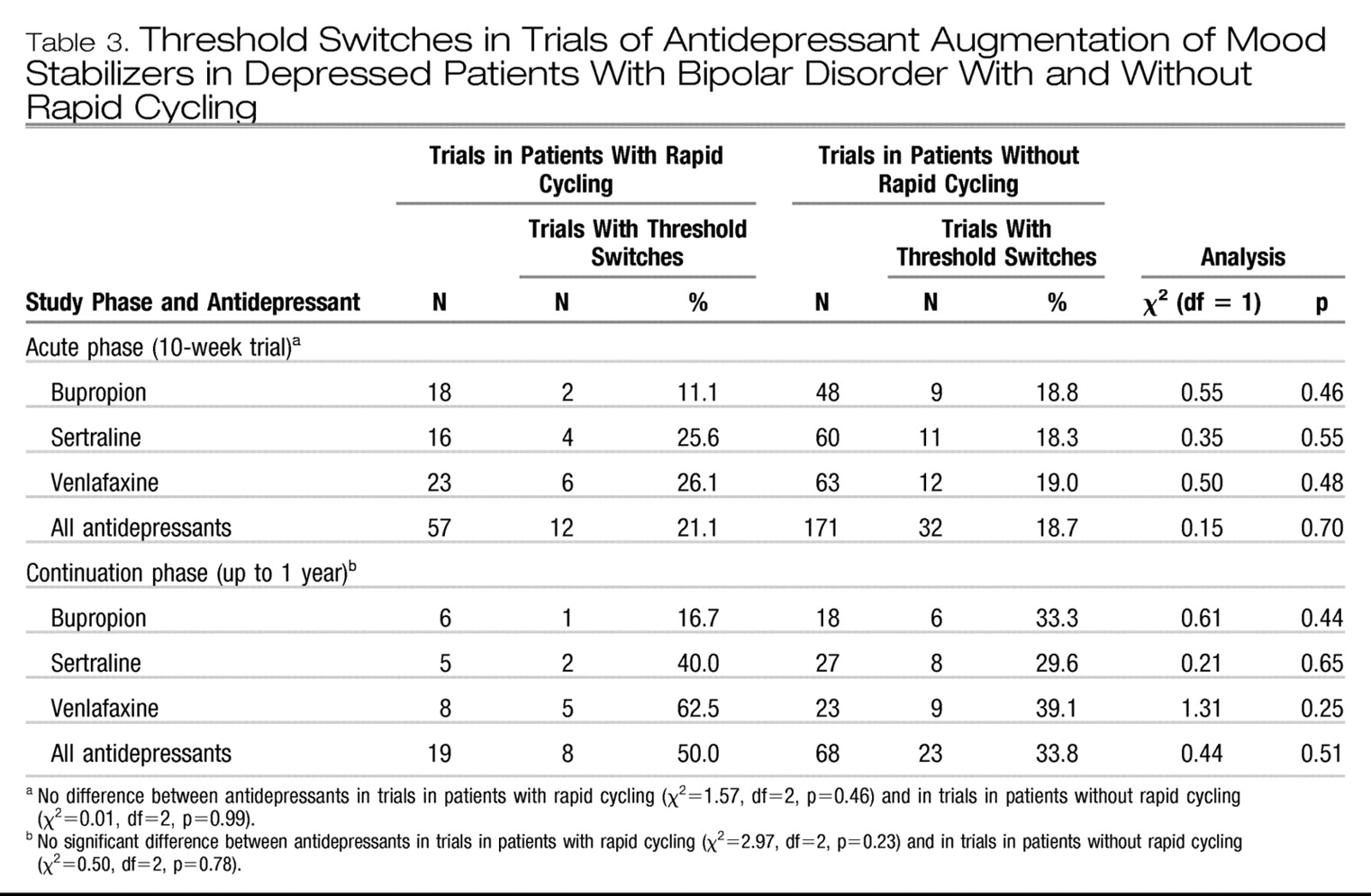
As Table 3 shows, more switches occurred in the rapidcycling group than in the non-rapid-cycling group, although none of the differences were significant. In the small number of trials including rapid-cycling patients in the continuation phase, the switch rate was 16.7% for bupropion, 40% for sertraline, and 62.5% for venlafaxine.
Discussion
We found a continuum of hypomanic to manic manifestations during antidepressant augmentation of mood stabilizers in patients with bipolar depression. The continuum ranged from sub-threshold (brief and recurrent brief) hypomania to threshold switches, including full-duration hypomania and mania associated with some dysfunction. These findings from the assessment of a range of switch thresholds in the same study may help explain the very different switch rates reported in previous studies in the literature. If only switches into mania (as defined in this study) are considered, then the switch rate of 7.9% in the acute treatment trials (and 14.9% in continuation trials) in this cohort would be consistent with rates found in many other acute treatment studies (
2,
7,
8). If one also considers full-duration hypomania (lasting a week or more) as a switch (even though many patients and clinicians would not see these extended hypomanias as clinically problematic), then a threshold switch occurred in 19.3% of the acute trials and 36.7% of the continuations trials. The switch rate that we found in the continuation trials is similar to the 35% switch rate that was deemed likely to be related to use of an antidepressant in a previous uncontrolled study in which retrospective life charts were used (
22).
The current study revealed a lower risk of switching in patients with bipolar II disorder than in patients with bipolar I disorder in the acute as well as the continuation trials. These findings extend those of Altshuler et al. (
23), who examined data from the acute treatment phase only and used the Young Mania Rating Scale and CGI-BP criteria for a switch.
In the continuation phase, the switch rate was almost twice as high as that observed in the 10-week acute phase, as might be expected on the basis of the much longer period of observation. Subtracting the number of continuation trials associated with a switch from those with an antidepressant response yielded a 42.5% overall response rate (37 of 87) in the evaluable group that entered the continuation phase. However, only these 37 (16.2%) remained from the original 228 acute intent-to-treat antidepressant trials, or 23.3% of the patients had a long-term antidepressant response without a switch into hypomania or mania in both phases.
Individual Drug Switch Rates
In the cross-sectional analysis of the acute treatment phase in which the Young Mania Rating Scale or CGI-BP severity cutoff scores were used to identify switches in this same cohort, venlafaxine also had a significantly higher switch rate, compared with bupropion or sertraline (
9). Those findings are consistent with the current observations (Figure 3) of a threefold higher ratio of the two types of full switches to the two types of subthreshold brief hypomanias during treatment with venlafaxine, compared to bupropion, and a more than twofold higher ratio with venlafaxine, compared to sertraline.
These data support and extend the findings of Vieta et al. (
24) that the combined reuptake inhibitor venlafaxine was associated with a greater risk of a switch, compared to another serotonin selective antidepressant, paroxetine. This difference was likely to be related to the additional noradrenergic actions of venlafaxine. This possibility is also suggested by the findings of Sachs et al. (
17,
18), who observed more switches in patients taking the potent noradrenergic reuptake inhibitor desipramine than in patients taking bupropion, which increases dopamine in the striatum and has weaker effects on norepinephrine.
In the cross-sectional analysis of the data from the acute phase (
9), rapid-cycling patients showed a higher switch rate while taking venlafaxine, compared to the other drugs. In the current analysis of both the acute and continuation antidepressant trials, the switch rates in trials in patients with a history of rapid cycling were not significantly different from the rates in trials in patients without rapid cycling, although venlafaxine was associated with the highest and bupropion with the lowest switch rate in both the acute and continuation phases in patients with rapid cycling. These findings not only highlight the likely higher risks associated with venlafaxine (
9,
24) but also support initial open observations (
25) of a relatively low risk of switches with bupropion, even in patients with rapid cycling, when it was given in combination with a mood stabilizer such as lithium.
Study Limitations
Several methodological factors limit the interpretation and inferences drawn from this study. The generalizability of these data from this outpatient cohort to other nonacademically based populations of patients with bipolar illness remains uncertain. Twenty-five percent of the study patients had a past history of rapid cycling. Although this rate of rapid cycling is similar to that reported for other cohorts (
26), patients with rapid cycling are typically excluded from antidepressant studies reported in the literature (
2), and such exclusions may yield lower switch rates in those studies. However, patients with rapid cycling were included in an earlier open-label trial (
27) and represented 22.1% of the population in a controlled trial of quetiapine in acute depression (
28). In the study of lamotrigine by Calabrese et al. (
29), 28% of the recently depressed patients had rapid-cycling bipolar disorder. These data suggest that the proportion of patients with rapid cycling in this study of acute depression was not atypical.
Twenty-five (13%) of the original 184 patients in the acute randomization (
9) did not have adequate life chart data to allow ratings of antidepressant improvement or switches in the current study. However, there was no evidence that these 25 patients, for whom other cross-sectional ratings were available, differed significantly from those with life chart data that were suitable for analysis.
Another possible limitation of this study is the fact that some patients in the acute phase were randomly assigned to receive a second or third antidepressant if they did not respond to the first, and the current analyses include data for patients who were and were not previously exposed to another antidepressant. Analyzing the data for the three randomizations separately by survival analysis revealed that receiving a drug in the first randomization, versus a later (second or third) randomization, did not make a significant difference in either the response or switch rates. Thus, we included data from both the first and the later randomized trials in the current analysis for simplicity of presentation and to allow assessment of the highest number of continuation trials of a given drug in order to have the greatest power to detect differences among the three drugs.
At the same time, data from the rerandomization, which involved 69 of the first 159 patients assigned to receive an antidepressant, yielded additional clinical information. As in the observations of Sachs et al. (
18), we found a lack of consistency for switching in the same individual during treatment with various second-generation antidepressants with different mechanisms of action.
Finally, in the absence of a placebo group, it was not possible to differentiate the switches directly related to antidepressant treatment from those related to the natural course of bipolar illness.
Clinical Implications
The clinical implications of the current findings are highly suggestive but not definitive. They support the view that in an academically based outpatient cohort of patients with bipolar disorder, anti-depressant augmentation, in general, is not likely to yield a high rate of sustained antidepressant response without a switch throughout both the acute and continuation treatment phases (
1,
5). Although the risk for switching in patients with bipolar I disorder was approximately linear for the first 75 days of antidepressant augmentation for all three drugs (Figure 2), thereafter it continued on this same steep trajectory for venlafaxine but became more gradual for bupropion. For sertraline, the slope was intermediate (closer to that for venlafaxine), and then no further switches were seen after day 160.
Given that naturalistic studies show that patients with bipolar disorder spend a threefold greater amount of time depressed than manic (
30–
33), even when antidepressants are used liberally, it appears that alternatives to adjunctive treatment with antidepressants are needed (
34–
37).
In a study of one alternative, Young and colleagues (
7) found less than satisfactory response rates both for the addition of an antidepressant to a mood stabilizer and for the addition of a second mood stabilizer. Given the promising antidepressant effects of lamotrigine (
29,
38) and some of the atypical antipsychotics in initial studies (
28,
39,
40), it may be useful to conduct direct randomized comparisons of augmentation of a mood stabilizer with an antidepressant to that with lamotrigine or an atypical antipsychotic (
13,
37) in instances of breakthrough bipolar depression.