ADDICTION IS A BRAIN DISEASE
Although the initial use of any substance is a voluntary choice, with continued administration of large amounts of that substance, that choice becomes progressively less voluntary and more influenced by the brain's adaptation to the chronic presence of the substance. This adaptation appears to create a drive to maintain the substance-induced homeostasis; a drive that uses brain mechanisms to see, hear, smell, or otherwise identify a cue that may lead to obtaining the substance. Numerous studies have shown the impact of chronic, long-term administration of drugs of abuse in both human and animal models (
30). Changes in the quantity and functionality of neurotransmitter receptor systems and the quantity of the neurotransmitters themselves eventually yield a state of
physiological dependence, a state marked by a biologically based drive for the exogenous substance of abuse to maintain this new homeostasis. Failure to fulfill that drive can cause the organism to experience
withdrawal, an unpleasant state marked by specific symptoms that vary according to substance but are relieved by that drug or a close substitute; thus, in conditioning terms, the alleviation of withdrawal symptoms provides
negative reinforcement (reinforced behavior due to the removal of a negative stimulus) for continued use of a drug. Physiological dependence is one of the key factors driving nicotine, alcohol, and opioid dependence. Even when an individual is detoxified from a substance, craving for that substance can lead to a return to drug use. Craving can be triggered by stress, environmental cues (
31), or small amounts of the drug itself (
32). Thus, another goal of addiction psychopharmacology is to minimize the craving that can lead to relapse.
REPLACEMENT THERAPY—FULL AGONISTS
The withdrawing brain does not know what chemical may be binding to and stimulating its receptors—e.g., in the case of heroin withdrawal, little distinction is made between heroin and methadone—both will relieve the body aches, nausea, diarrhea, anxiety, and elevated pulse that comprise opioid withdrawal in general. That is, the
pharmacodynamics of heroin and methadone are similar: they have similar activity at the μ-opioid receptor. However, they are different in several important ways. Methadone is less potent, has a higher affinity, and its effects last longer at the μ-opioid receptor than heroin (its half-life is 36 hours compared with 1 hour for heroin). This is the basis of replacement therapy: a drug of abuse can be replaced with a medication that has a longer duration of action, less abuse potential, and a better safety profile to prevent drug withdrawal and craving. Indeed, many studies support the use of methadone treatment for heroin and other opioid dependence because it reduces incidence of HIV infection (
33), intravenous drug use (
34), unsafe sex (
35), and crime (
36). An active heroin user costs society roughly
10 times that of a person in a methadone treatment program (
37).
Similarly, replacement therapy has been used with success in tobacco dependence as well, in the form of nicotine replacement therapy (
38). In this case, the active chemical in both the substance of abuse and the medication being taken is the same: nicotine. The key difference between tobacco and nicotine replacement therapy is the exposure to carcinogens and other aromatic hydrocarbons that carry with them risks of developing cancer, lung disease, heart disease, and many other serious illnesses. The most common form of nicotine replacement therapy is the nicotine patch, which, analogous to methadone, ensures the delivery of a constant supply of the chemical the dependent brain craves, while eliminating most of the risk associated with the drug of abuse, in this case, tobacco (
39). Other, more rapidly acting forms of nicotine replacement therapy, include nicotine gum, lozenges, nasal spray, and inhalers.
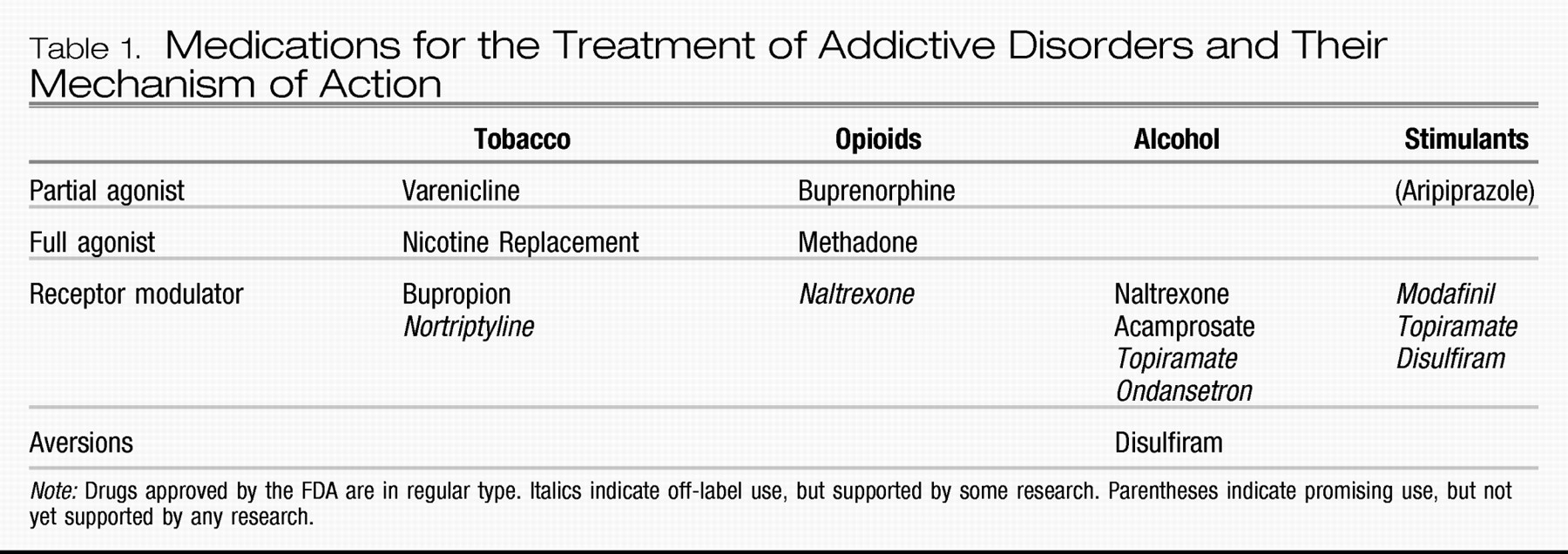
REPLACEMENT THERAPY—PARTIAL AGONISTS
Opioid dependence. In contrast to full agonists such as methadone and nicotine, which are chemicals that bind to a class of receptors and stimulate them fully to produce their effects, there exists a class of medications that both stimulate and block brain receptors. These partial agonists, newly approved by the U.S. Food and Drug Administration (FDA), are available to treat opioid and nicotine dependence (see discussion below). The effects of full agonists such as methadone are the same as those typically seen with opioids, i.e., sedation, euphoria, constipation, analgesia, tolerance, and, unfortunately, respiratory depression. The latter effect means that use of methadone carries with it a real risk of overdose, especially when it is combined with other sedating drugs, such as benzodiazepines (
40). Because of this and other risks (e.g., diversion of prescribed methadone for sale on the street), methadone can only be legally prescribed for the treatment of opioid dependence by federally licensed methadone clinics (it can, however, be prescribed on an outpatient basis for the treatment of pain).
Buprenorphine is a
partial agonist at the μ-opioid receptor—i.e., it binds to the same receptors as other opioids but only
partially stimulates the receptors. In the literature, this action is sometimes referred to as an
agonist-antagonist effect. The partial μ-opioid receptor stimulation does treat craving for opioids. However, because it is only
partial stimulation, there is a limit to its opioid effect, called the “ceiling effect” (
41), even at 100% μ-opioid receptor saturation (
Table 1). This limit to its μ-opioid activity allows buprenorphine to have a much better safety profile, and patients report better functioning because of the absence of sedation seen with methadone and other full agonists. The high affinity of buprenorphine for μ-opioid receptors also diminishes the
positive reinforcement that taking any additional opioids will provide (
42). As a result of this improvement in safety profile and abuse liability, the federal government designated buprenorphine as the first medication available to treat opioid dependence on an outpatient basis. By taking an 8-hour course, physicians can qualify for a waiver, under the Drug Addiction Treatment Act of 2000 (DATA 2000), which allows them buprenorphine prescription privileges.
An important note about buprenorphine is that it should only be administered initially when the patient is in withdrawal, so that it will improve withdrawal symptoms. If given to an opioid-dependent person who has enough full-agonist opioids on the receptors, the higher-affinity buprenorphine will displace the full agonist, immediately precipitating withdrawal (
42). Therefore, it is recommended that induction onto buprenorphine be done in a physician's office with frequent assessment of withdrawal using a validated scale such as the Clinical Opioid Withdrawal Scale.
The availability of several preparations of buprenorphine is a frequent point of confusion among the lay public and physicians alike. The trade names for buprenorphine (sometimes called “bup”) preparations are Subutex and Suboxone. Both are pills taken sublingually that come in 2- and 8-mg sizes, and the dose given is usually between 2 and 32 mg/day. Subutex contains only buprenorphine; Suboxone also contains naloxone, a medication that is only active parenterally. The naloxone is included to prevent Suboxone from being ground up, dissolved, and injected, a method sometimes used by those seeking to maximize the speed of onset and effects of a drug. When taken sublingually, as directed, the naloxone has no activity; however, if injected, the naloxone will block the effects of buprenorphine and, in an opioid-dependent individual, will precipitate an immediate and unpleasant opioid withdrawal (
43).
Nicotine dependence. Varenicline is a partial agonist approved by the FDA in August 2006 for the treatment of nicotine dependence. It is active at the α
4β
2 nicotinic acetylcholine receptor, the same receptor activated by nicotine itself. A recent study showed it to be significantly more effective than bupropion for the treatment of nicotine dependence (
44). It does not precipitate the same intense withdrawal that buprenorphine can, and, therefore, it can be titrated upward while the patient is still smoking. After 1 week, when the full dose of 1 mg twice daily has been reached, the patient will find his or her craving for nicotine reduced and the pleasurable effects of nicotine blocked because the higher-affinity varenicline is binding to the α
4β
2 receptors (
44).
Stimulant dependence. Aripiprazole is an FDA-approved medication indicated for use in schizophrenia and bipolar disorder, which, as a partial D
2 receptor agonist, has attracted some interest as a potential treatment for cocaine and methamphetamine dependence. Although it has been shown to reduce some of the pleasurable effects of
d-amphetamine (
45), there have not as yet been any studies showing a decrease in methamphetamine use. There is also no evidence of any efficacy for decreasing cocaine use.
RECEPTOR MODULATORS
Nicotine dependence. Receptor modulators are the other mainstay of addiction psychopharmacology. The first medication to gain FDA approval using the mechanism of receptor modulation was bupropion. The antidepressant efficacy of bupropion is thought to occur via its inhibition of norepinephrine and dopamine reuptake. Because of the importance of dopamine in the maintenance of addictive behaviors, inhibition of dopamine uptake is also thought to be a key mechanism in its action as an anti-nicotine craving agent (
46). Interestingly, it also exhibits some antagonism at the α
4β
2 nicotinic acetylcholine receptors, which may also play some role in its anticraving properties (
46). Dosing for bupropion for addiction is equivalent to that for its use in depression.
Alcohol dependence. Several treatments are available for the treatment of alcohol dependence. The first to be approved by the FDA was disulfiram in 1948. Disulfiram is an aldehyde dehydrogenase inhibitor, which allows the buildup of acetaldehyde, the by-product of alcohol metabolism responsible for the symptoms of hangover with which most drinkers are familiar: nausea, headache, flushing, and so on. The psychological effect at play is
punishment, and, consistent with learning theory, it is not a very good way to extinguish drinking behavior. Better results are obtained with disulfiram with supervision by a spouse (
47) and motivated populations (
48). The serious side effects of hepatotoxicity and cardiovascular effects can be mitigated by using lower doses, such as 125–250 mg daily (
48).
Almost 50 years later, in December 1994, the second medication for the treatment of alcohol dependence was approved by the FDA. This medication is the oral form of naltrexone which is usually started and maintained at 50 mg/day. Starting the medication at a half-dose and then titrating up to the full dose can reduce its minor gastrointestinal side effects (
49). In April 2006, a monthly depot injectable form of naltrexone was approved. This was an exciting development because poor treatment response to oral naltrexone is usually due to poor medication adherence (
49). Depot naltrexone is given at a dose 380 mg i.m. per month and is safe and effective, even in the 1st month (
50). Naltrexone is an antagonist at the μ-opioid receptor and is thought to reduce drinking by blocking the craving for and euphoria from alcohol consumption (
51). Several double-blind, placebo-controlled trials of naltrexone have demonstrated that naltrexone increases the time to relapse and decreases alcohol consumption and the quantity and frequency of drinking among alcoholics who relapse (
52).
Acamprosate gained FDA approval for the treatment of alcohol dependence in July 2004, although it has been used in Europe for more than 10 years. Acamprosate is thought to have its anticraving efficacy as a partial agonist at the
N-methyl-
d-aspartate receptor (
53), a key receptor system that is dysregulated in alcohol dependence (
54). The effectiveness of acamprosate in helping patients maintain abstinence from alcohol is supported by several randomized, controlled, clinical trials, reviewed in a meta-analysis by Mann et al. (
55). Unfortunately, in a recent large multisite trial comparing it to naltrexone and the combination, acamprosate did not separate from placebo (
56), although questions have been posed as to whether the study design allowed the effectiveness of acamprosate to be recognized (
57). It is given at a dose of 666 mg three times daily. Side effects are mainly mild gastrointestinal symptoms, such as nausea, diarrhea, and flatulence. Other receptor modulators showing some positive data include topiramate (
58) and ondansetron (
59), although these have not yet been approved by the FDA.
Stimulant dependence. No medications are FDA-approved for the treatment of dependence on stimulants, e.g., cocaine and methamphetamine. However, modafinil (
60), topiramate (
61), and disulfiram (
62) did show some positive results in pilot trials in cocaine dependence. Because the mechanism of action of the drugs and withdrawal phenomena are similar, results from cocaine trials are hopeful for methamphetamine dependence. Whereas cocaine inhibits reuptake of dopamine and norepinephrine, methamphetamine inhibits reuptake and directly stimulates the release of these catecholamines.
Modafinil is an FDA-approved treatment for somnolence associated with narcolepsy, obstructive sleep apnea, and shift-work sleep disorder. It is thought to blunt cocaine euphoria and increase abstinence via its glutamate-enhancing action and dopamine reuptake inhibition. In their study of 62 cocaine-dependent patients, Dackis et al. (
60) found that subjects randomly assigned to modafinil 200 mg b.i.d. had significantly more clean urine drug screens and were more likely to have prolonged abstinence than those taking placebo. A larger study is currently underway to confirm these results.
Topiramate is an anticonvulsant medication that has γ-aminobutyric acid-agonist activity and inhibits glutamatergic neurotransmission, neurotransmitter systems thought to be involved in the rewarding effects of stimulants. Kampman et al. (
63) have published pilot data on the efficacy of 200 mg/day of topiramate versus placebo in 40 cocaine-dependent patients. Topiramate-treated patients were more likely to be abstinent from cocaine and more likely to sustain three continuous weeks of abstinence than patients treated with placebo. Kampman et al. are also conducting a larger randomized, placebo-controlled trial of topiramate for the treatment of patients with both cocaine and alcohol dependence.
In addition to its properties as an aldehyde dehydrogenase inhibitor, disulfiram increases dopamine levels through an indirect mechanism, which could explain its efficacy in reducing cocaine use (
62).