Abnormal motor behaviors, including slowed movement, shuffling gait, collapsed posture, low voice volume, monotone speech, lack of facial expressivity, and purposeless movements of the limbs and trunk, were recognized as a fundamental feature of depressive disorder by the earliest psychiatric writers, including Aretaeus of Cappadocia, Hippocrates, Caelius Aurelianus, and Plutarch.
1,2 Because the thought and will of many patients also seemed impaired, the altered motor and psychic components of depression were jointly described as the “psychomotor” symptoms of depression. They have continued to be a cardinal feature of depressive illness in all of the major contemporary psychiatric classification systems.
3 Most recently, increasing recognition of the neurologic aspects of depressive disorder
4 has aroused new interest in the potential neuropathologic significance of psychomotor symptoms in depression.
5 The high rates of mood disorder that occur in the context of neurologically based motor disorders such as Parkinson's disease,
6 supranuclear palsy,
7 Huntington's disease,
8 Meige's syndrome,
9,10 and Wilson's disease
11,12 have led some to propose that the well-defined neuropathology of specific basal ganglia diseases may provide a useful model for investigating the pathophysiology of mood disorders with motor impairment.
13 Recent brain imaging findings provide initial support for this approach. SPECT studies comparing severely depressed patients with an age-matched normal comparison group revealed decreased regional cerebral blood flow (rCBF) in paralimbic regions, including the inferior frontal, anterior cingulate, and anterior temporal cortex.
14 When the association between psychiatric symptoms and perfusion was examined, only clinically rated psychomotor slowing was correlated with perfusion levels. In another study, clinically assessed psychomotor retardation was associated with reduced rCBF in the left dorsolateral prefrontal cortex and left angular gyrus, whereas increased blood flow in the cingulate cortex and inferior parietal lobe correlated with psychomotor agitation.
15 Segregated basal ganglia projections innervate the dorsolateral prefrontal cortex, the anterior cingulate region, and the lateral temporal lobe.
16 These findings are thus consistent with a theory of depression which posits that abnormalities in basal ganglia–thalamocortical circuitry may underlie its psychomotor manifestations.
14Psychomotor phenomena in depression, however, have yet to be clearly defined. Thus, the investigation of the possible neuropathologic significance of psychomotor symptoms in depression is seriously limited.
17 The relative contribution of motor and cognitive components to psychomotor disturbance is unknown.
18 This lack is reflected in the current psychiatric criteria, where the term
psychomotor symptoms refers to virtually any manifestation of slowing or restless activity during a depressed state (DSM-IV). Clinical rating scales of depression typically include only one item for psychomotor disturbance; “agitation” and “retardation” may be combined; and cognitive and motor aspects of agitation and retardation are intermixed. The rates of observable motor symptoms in depressed patients have been infrequently reported, and manifestations of motor disturbance believed to differentiate depressed patients have rarely been clinically assessed in normal control subjects. Furthermore, the two previously published rating scales that were constructed to assess psychomotor symptoms in depression, the Retardation Rating Scale (RRS)
19 and the CORE,
20 combine motor and cognitive symptoms and omit several aspects of motor behavior cited in the literature as being associated with agitation and retardation in depression.
The Motor Agitation and Retardation Scale (MARS) was explicitly developed to assess the motor abnormalities associated with “psychomotor” disturbance in depression. MARS items were derived from published empirical studies of motor functioning in depressed patients and were intended to provide a comprehensive and nonredundant complement of motor abnormalities manifested during a depressive episode. The MARS uses an empirically based anchor point rating system and was designed to offer a rapid (10–15 minute) clinical assessment of 19 abnormal motor behaviors associated with clinically diagnosed depression.
METHODS
Subjects
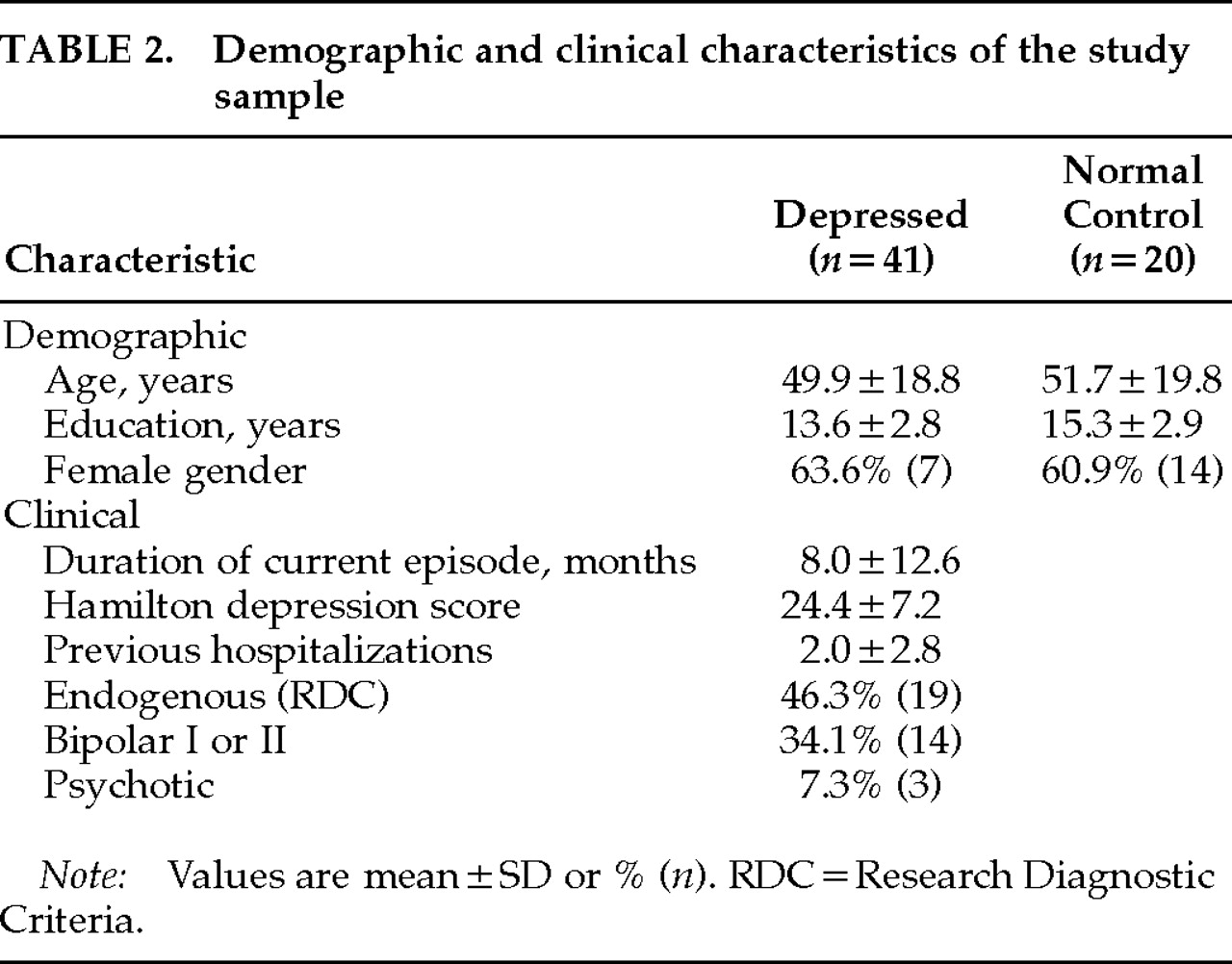
Patients in this study were consecutive admissions to a psychiatric ward and met criteria for DSM-IV major depressive episode, with lifetime diagnoses of either major depressive disorder (unipolar), bipolar I, or bipolar II. Fifty-two depressed inpatients and 20 normal control subjects were initially assessed, and reliability ratings were completed on a subsample of 21 depressed patients and all of the 20 normal control subjects. When ratings were complete, it was determined that 11 of the 52 depressed patients were taking neuroleptics at the time of testing. These patients were excluded from the analyses. Of the remaining 41 depressed patients, 17 (41%) were medication-free at the time of testing, 13 (32%) had been taking non-neuroleptic medications for 10 days or less, and 11 (27%) had been taking non-neuroleptic medications for longer than 2 weeks. The medications administered included mood stabilizers (anticonvulsants or lithium), antidepressants (SSRIs, MAOI, tricyclics, heterocyclics) and anxiolytics (benzodiazepine and non-benzodiazepine classes). One patient was also receiving a stimulant for augmentation purposes. The control sample was recruited from a normal control database fully screened and diagnosed prior to enrollment in the study. All normal control subjects were “never mentally ill” according to Research Diagnostic Criteria and had no history of neurologic disease or symptoms.
Scale Development and Description
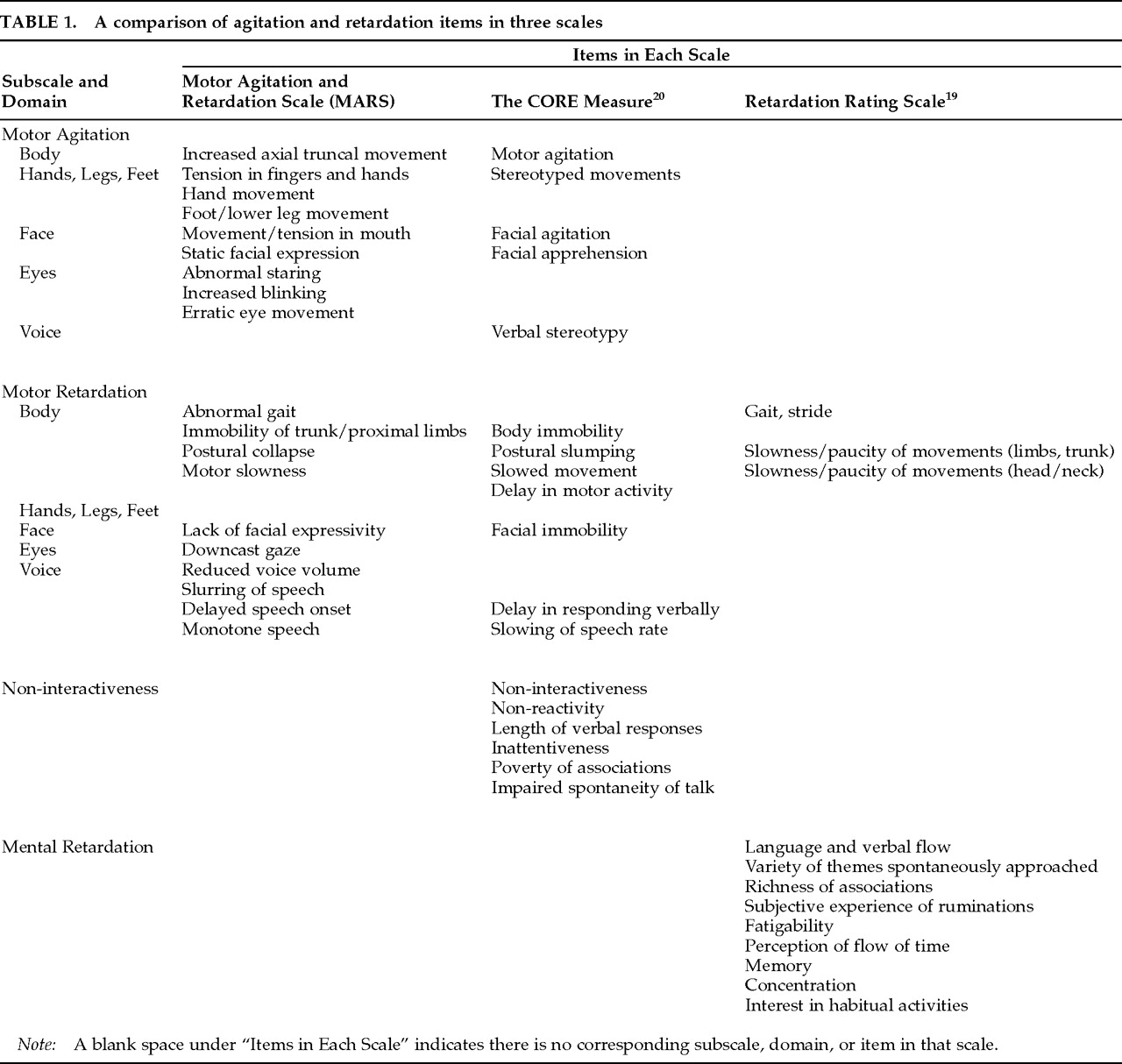
Only observable motor signs associated with agitation and retardation were included in the MARS. The empirical literature and clinical observation suggest that motor changes could be manifested in five domains, including trunk (“body”), limbs, eyes, face, and voice.
Table 1 summarizes these manifestations and compares them with the content of the two previously published motor scales. Two items, Erratic Eye Movement and Increased Blinking, were added because of their possible value as markers of caudate abnormality.
21 These two items are provided for the rating of readily visible darting, shifting, or jerking movements of the eyes (Erratic Eye Movement) and obviously abnormal blinking behavior (Increased Blinking). They are not intended to assess subtle alterations in eye behavior such as movements of the lids or saccadic irregularities.
22The MARS items are scored on a four-point scale. Pilot studies indicated that rater unreliability was often attributable to the inconsistent application of “severity” criteria, which logically could be rated according to either the frequency of a behavior or its magnitude. The anchor points were subsequently altered to reflect the criteria that were most appropriate for each item: none (1), rare (2), periodic (3), and continual (4) for discrete behaviors such as abnormal hand movements; and none (1), mild (2), moderate (3), and severe (4) for items describing pervasive phenomena such as motor slowness.
The MARS was designed to be used, with little to no additional training, by professionally trained clinicians who are experienced in treating depression and willing to adhere closely to the instructions and item definitions. Scale instructions were streamlined, brief behavioral descriptions were included in item definitions, and an administration manual was developed. Prior to this study, the MARS was piloted by four clinicians who completed ratings on 30 depressed patients participating in ongoing treatment efficacy studies. Two subsequent revisions were piloted by three raters using videotaped recordings of 65 inpatients who met either Research Diagnostic or DSM-III-R criteria for major depressive disorder.
Rating Procedure
Potential study participants were identified by attending ward physicians within the first 3 days of admission. The rating procedure was explained to patients and informed consent was obtained prior to the interview session. The raters for this study included a psychiatrist (L.M.) and a clinical psychologist (C.S.). Raters discussed MARS items and anchor points for approximately 60 minutes prior to the first rating session, and they discussed and compared ratings following each of the first 5 cases. Discussions of subsequent cases were infrequent. Raters were blind to DSM diagnosis and medication status at the time of the ratings. To control for diurnal variation, all ratings were completed between the hours of 8:00 a.m. and 1:00 p.m., with 95% of the ratings occurring between the hours of 8:00 a.m. and 10:00 a.m. Gait was assessed first by having the subject walk about 20 feet, toward the raters. Following this, a set of standard questions regarding basic demographic and symptom information was administered, and subjects were continuously observed for about 10 minutes. The MARS was scored immediately following the observation period. Two additional motor scales, the RRS and the CORE, were rated for 26 of the depressed patients.
RESULTS
Subjects
Depressed patients and normal control subjects did not differ with regard to age, gender, or education (
Table 2).
Comparisons by t-test of unmedicated and non–neuroleptic-medicated patients' MARS item ratings also revealed no differences. However, individual t-test comparisons (df=40,10) of MARS item ratings for the neuroleptic-treated and non–neuroleptic-treated patients revealed that patients taking neuroleptics at the time of assessment had significantly increased abnormal gait (P=0.04), lack of facial expressivity (P=0.04), monotone speech (P=0.03), and abnormal staring (P=0.05). Further comparisons revealed that patients treated with neuroleptics had significantly more previous hospitalizations, and, as expected, 9 of 11 neuroleptic-treated patients had psychotic symptoms. Neuroleptic-treated patients were excluded from further analyses.
Interrater Reliability
Interrater reliability, as assessed with intraclass correlations, ranged from excellent (Downcast Gaze, 0.92; Delayed Speech Onset, 0.86; Abnormal Gait, 0.85) to satisfactory (Immobility of Trunk, 0.63; Lack of Facial Expressivity, 0.62; Postural Collapse, 0.58; Slurred Speech, 0.52; Motor Slowness, 0.50) for all of the retardation items except Reduced Voice Volume (0.43) and Monotone Speech (0.32). With regard to agitation, raters achieved excellent to satisfactory agreement on the rating of Abnormal Staring (0.81), Movement and Tension in the Mouth (0.72), Foot and Lower Leg Movement (0.66), Tension in Fingers and Hands (0.61), and Abnormal Hand Movement (0.50). For four agitation items, intraclass correlations were <0.50 (Increased Axial Truncal Movement, Increased Blinking, Static Facial Expression, and Erratic Eye Movement). These individual correlations are reflected in the intraclass correlations for subscale and total scale scores: 0.87 for retardation items, 0.38 for agitation items, and 0.74 for full-scale scores. For items with intraclass correlations below 0.50, kappa coefficients were calculated. All kappas ranged between 0.45 and 0.70, indicating acceptable to good interrater agreement
23 and suggesting that the lower intraclass correlations were attributable to disagreement regarding the severity of items, rather than their presence. The individual item kappas indicated the relative rating difficulty associated with individual behaviors: Monotone Speech (0.45), Blinking (0.48), Erratic Eye Movements (0.49), Static Facial Expression (0.60), Voice Volume (0.70), and Increased Axial Truncal Movement (0.70).
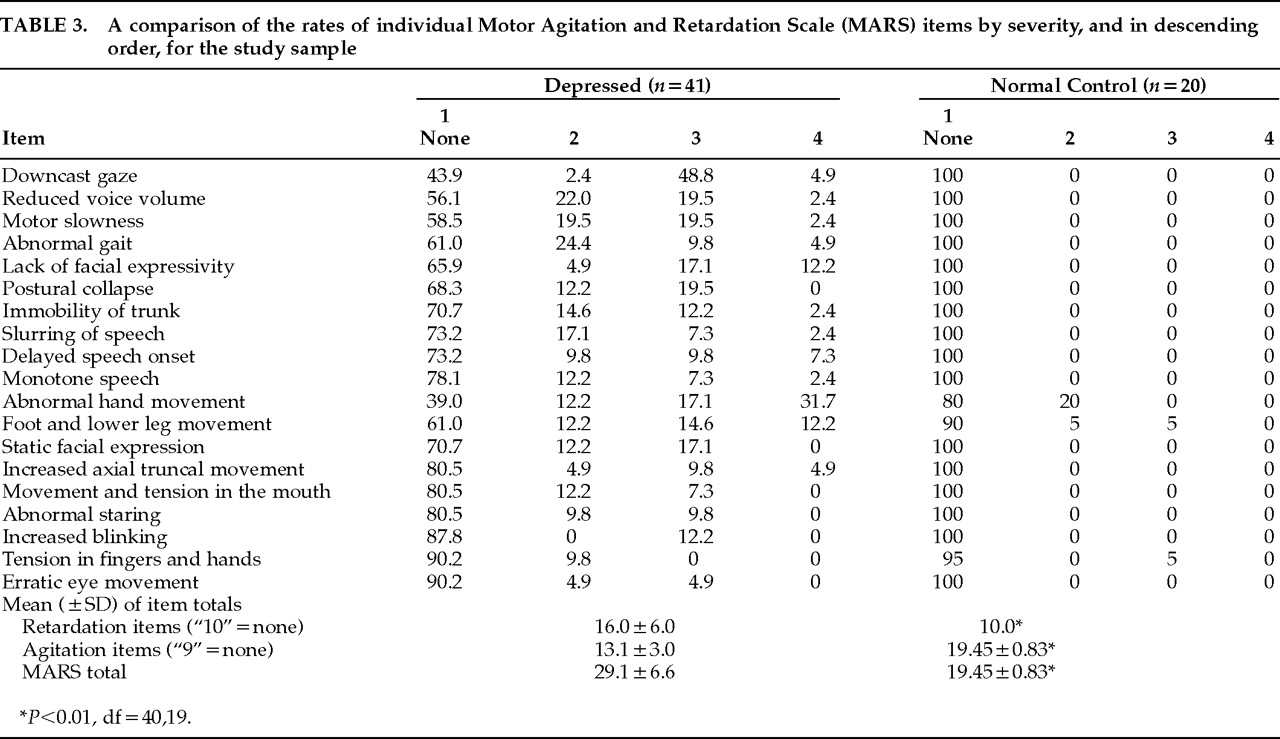
Item Performance and Sensitivity
Table 3 gives the rates of individual items in descending order by severity level. Depressed patients differed significantly from normal control subjects on retardation, agitation, and full scale scores (df=40,19,
P<0.01). The most frequently occurring motor abnormalities (>40% of cases) among depressed patients included Abnormal Gait, Downcast Gaze, Reduced Voice Volume, Motor Slowness, Abnormal Hand Movement, and Foot and Lower Leg Movement. Behaviors that were rated in less than 10% of patients included Tension in Fingers and Hands, Increased Blinking, and Erratic Eye Movements. Manifestations of motor abnormalities were absent in the normal control subjects with the exception of three agitation items that occurred infrequently, including Tension in Fingers and Hands, Abnormal Hand Movement, and Foot and Lower Leg Movement. Among the depressed patients, 1 of the retardation items and 6 of the agitation items were not observed at the most severe level defined on the scale.
Motor symptom profiles within patients revealed that approximately 10% of patients (n=4) had no motor symptoms. Another 10% had either 1 or 2 of the agitation symptoms that occurred infrequently among the normal control subjects, although in 2 of these patients, agitation abnormalities were manifested to a degree of severity not observed in normal subjects. Of the remaining patients, 75% (n=31) manifested symptoms of both agitation and retardation. Of these 31 patients, 11 (27%) had 3 to 5 motor symptoms, 15 (36%) had 6 to 9 motor symptoms, and 5 (12%) had more than 10 motor symptoms. The last 5% of the total patient group (n=2) showed no agitation abnormalities and had 8 to 9 retardation symptoms.
Internal Consistency and Item Analysis
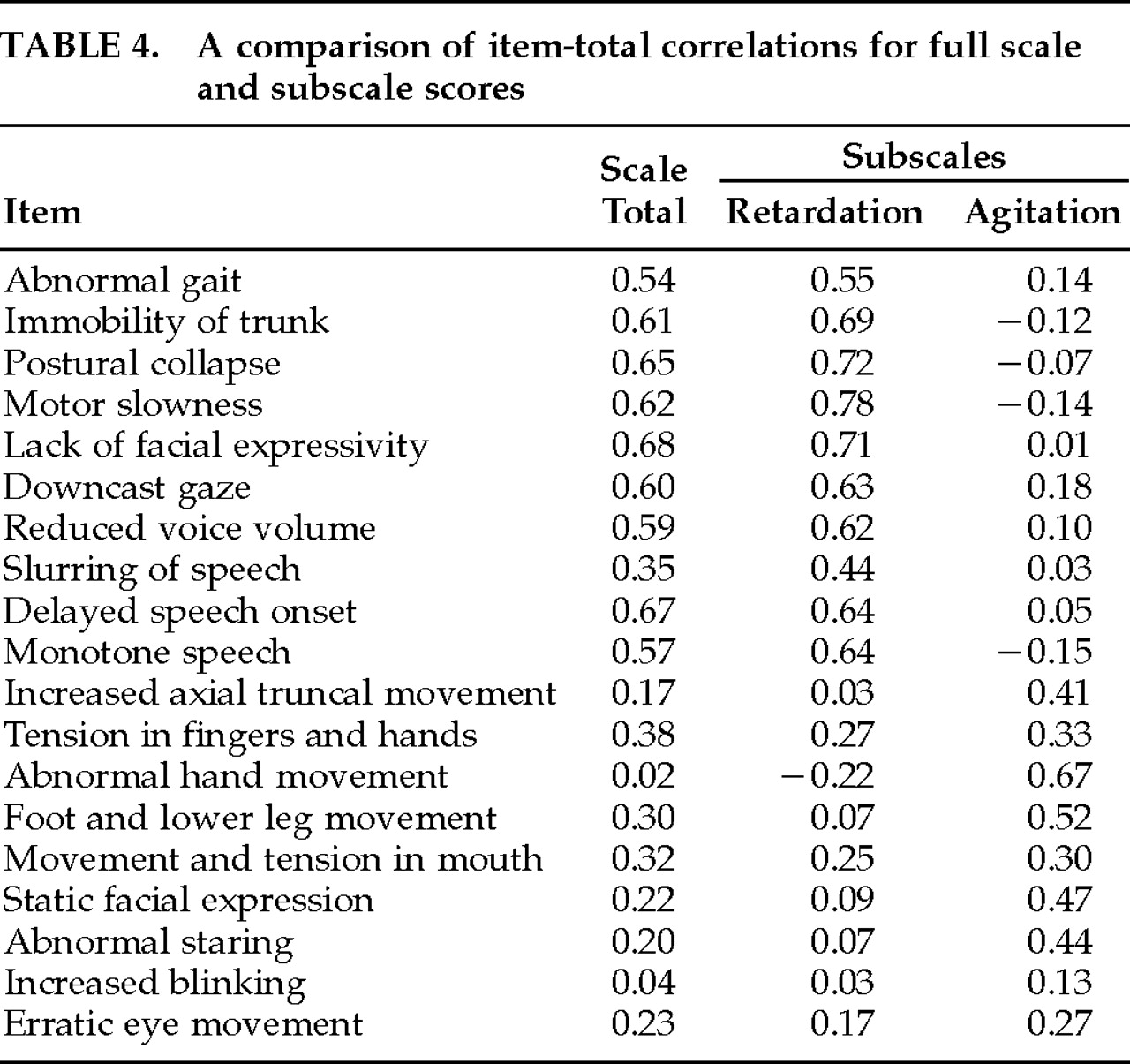
Cronbach's alpha coefficients were 0.83 for the full-scale MARS, 0.89 for the retardation subscale, and 0.68 for the agitation subscale. Extracted-item alphas ranged from 0.79 to 0.84 for the full-scale MARS, from 0.87 to 0.91 for the retardation subscale, and from 0.56 to 0.71 for the agitation subscale. All of the retardation items were adequately associated with the full-scale total, except Slurred Speech (
Table 4). Item-total correlations with the retardation subscale score ranged from 0.44 to 0.72. None of the retardation items correlated even moderately with the agitation subscale total. The retardation subscale total score correlated 0.84 with the MARS total.
Overall, the agitation items appeared to contribute less to the full-scale total, with only 3 items correlating ≥0.30 (Tension in Fingers and Hands, Movement and Tension in the Mouth, and Foot and Lower Leg Movement). The agitation subscale total correlated 0.39 with the MARS total. Item-total correlations between agitation items and the agitation subscale total were more consistent: 7 of 9 items ranged between 0.30 and 0.67. Increased Blinking and Erratic Eye Movement appeared to contribute little, although this is likely due to their infrequency in this sample. None of the agitation items correlated even moderately with the retardation subscale score. The correlation between the agitation and retardation subscale scores was 0.02. Overall, the alpha levels suggested good to excellent internal consistency for the full scale and the retardation subscale and acceptable internal consistency for the agitation subscale. However, the lack of correlation between the agitation subscale and the total MARS, and between the two subscales, suggests that the subscales should not be combined into a single total score.
Validity
Twenty-six depressed inpatients were rated on the two previously published motor scales, the RRS
19 and the CORE,
20 for the purpose of assessing convergent validity. Total score correlations were not appropriate because both scales included several nonmotor items. To assess the validity of the MARS, matching RRS and CORE items were individually correlated. The RRS items Gait, Slowness and Paucity of Movement, and Voice Modulation were strongly correlated with the MARS items Abnormal Gait, Motor Slowness, and Reduced Voice Volume (
r2=0.72, 0.86, and 0.64, respectively). Six items on the CORE closely matched MARS items and were strongly correlated, including Facial Immobility and Lack of Facial Expressivity (0.92), Postural Slumping and Postural Collapse (0.94), Delay in Verbal Responding and Delayed Speech Onset (0.96), Facial Agitation and Static Facial Expression (0.94), Body Immobility and Immobility of Trunk (0.96), and Slowed Movement and Motor Slowness (0.94). The high correlations between the individual CORE and MARS items are likely attributable to the conceptual conformity of these items and the use of identical scale points.
To further assess the concordance between CORE and MARS ratings, CORE subscale scores were calculated separately for motor retardation items (Facial Immobility, Postural Slump, Delay in Verbal Responding, Slowed Speech), motor agitation items (Facial Apprehension, Facial Agitation, Motor Agitation, Stereotyped Movements), combined motor agitation and retardation items, and nonmotor non-interactiveness items (Non-Interactiveness, Non-Reactivity, Length of Verbal Responses, Inattentiveness, Poverty of Associations, Verbal Stereotypy, Impaired Spontaneity of Talk). The CORE and MARS motor retardation subscales were strongly correlated (r2=0.92), as were the combined CORE motor agitation and retardation items and the total MARS score (r2=0.74). The somewhat lower combined score correlation likely reflected the 5 additional agitation-related behaviors included in the MARS. This divergence is further indicated by the low correlation between the CORE and the MARS motor agitation subscale scores (r2=0.31). Discriminant validity was indicated by a lack of correlation between MARS agitation subscale score and the CORE motor retardation subscale score (r2=0.09), and between the MARS retardation subscale score and the CORE motor agitation subscale score (r2=0.02). The CORE nonmotor non-interactiveness subscale scores were uncorrelated with the MARS agitation subscale (r2=0.01) and were only weakly correlated with the MARS retardation subscale (r2=0.29). Correlations between Hamilton Rating Scale for Depression scores and MARS retardation subscale, agitation subscale, and total scale scores suggested that MARS scale scores were not simply a redundant measure of depression severity (r2=0.10, 0.14, and 0.21, respectively).
A multiple regression analysis was conducted to determine the association of demographic and clinical variables with the manifestation of motor abnormalities in depression. Neither gender, age, nor illness duration was associated with MARS total or subscale scores. A second regression was conducted to assess the relative association of depression subtypes with MARS ratings. RDC endogenous depression was identified in 46% of the sample, bipolar I depression was identified in 17%, and 7% of patients not treated with neuroleptics were psychotic. In this analysis, only endogenous depression was significantly associated with MARS total scale scores (df=37,3, P<0.01, F=20.9) and retardation subscale scores (df=37,3, P<0.001, F=12.4). In this analysis, there were no associations between any of the depression subtypes and the agitation subscale scores.
DISCUSSION
The MARS was developed to increase the total number of items available for the rating of motor disturbance in depression and thereby provide a scale with which to assess a full complement of motor abnormalities associated with agitation and retardation in depression. Seven items not included in the previously published motor scales were reliably rated, including Downcast Gaze, Slurred Speech, Abnormal Staring, Movement and Tension in the Mouth, Foot and Lower Leg Movement, Abnormal Hand Movement, and Tension in Fingers and Hands. In addition, the interrater reliability for MARS items approximated, and for some items improved upon, those for equivalent CORE items,
20 including Immobility of Trunk and Body Immobility (0.63 and 0.66), Postural Collapse and Postural Slumping (0.58 and 0.39), Lack of Facial Expressivity and Facial Immobility (0.62 and 0.47), Delayed Speech Onset and Delay in Verbal Responding (0.86 and 0.71), and Motor Slowness and Slowed Movements (0.50 and 0.67). Six items with low intraclass correlations had acceptable kappa coefficients, suggesting that raters disagreed on the severity rather than the presence of behaviors.
Several factors likely contributed to the raters' difficulty in agreeing on the severity of some items. Behaviors associated with agitation occurred sporadically and gave raters little opportunity to become practiced in their assessment. The validity of the items Erratic Eye Movements and Increased Blinking awaits further investigation in a sample of patients with higher rates of agitation. The manual descriptions and criteria for four items (Reduced Voice Volume, Monotone Speech, Increased Axial Truncal Movement, and Static Facial Expression) were altered in an attempt to improve reliability on these items in future studies. The interrater reliability of the agitation items may be artificially reduced because of a restricted range of agitation item scores.
Overall, normal control subjects did not manifest the motor behaviors represented on the MARS that were associated with agitation and retardation in depression. Although hand and leg movements occurred in a few normal control subjects, the distribution of ratings in depressed patients and normal control subjects differed markedly. At the same time, these findings suggest that the occurrence of purposeless hand and/or leg movements in the absence of other motor abnormalities (observed in approximately 10% of depressed patients) may be clinically insignificant. The rates of agitation and retardation reported in past clinical studies using single or dual item ratings have ranged between 46% and 67%,
24–27 whereas in this sample of depressed inpatients motor abnormalities were manifested in 75% of cases. The MARS is at least as sensitive as the most broadly cast single and dual item ratings. The diagnostic and clinical significance of the varying degrees of severity, and the striking overlap among the individual components of motor agitation and retardation, await further investigation.
Past studies have suggested that both gender and age significantly influence the manifestation of psychomotor symptoms. Males have been found to have more retardation than females; females have been thought to manifest more agitation than males.
28–30 Previous studies have also suggested that depressed patients under 40 are more likely to have motor retardation, whereas patients over 40 are more likely to have motor agitation.
28,30 None of these effects were apparent in this sample of 41 depressed inpatients when a full range of motor manifestations was assessed. The motor differences identified in neuroleptic-treated patients substantiated the importance of separating neuroleptic-treated patients in studies of motor symptoms. However, whether these differences were associated with psychosis, or with the nature of depressive disorder in neuroleptic-treated patients, or directly with effects of neuroleptic medications cannot be determined on the basis of these data. Echoing the findings of Parker and Hadzi-Pavlovic,
20 the endogenous subtype was strongly associated with MARS full-scale and retardation subscale scores.
Overall, the retardation items were reliable and internally consistent, and they contributed equally to the item totals. The agitation items fared less well. We reserve judgment regarding the contribution of agitation to the motor abnormalities in depression, however. Agitation behaviors were less frequent than retardation behaviors, and this sample was not enriched with agitated patients in order to increase the frequency of agitation item ratings. The relatively low variability of item scores restricted the intraclass correlations and may have resulted in underestimated interrater reliabilities for these items. We recommend that investigators assess a full range of agitation-related behaviors. Total MARS scores were used in the statistical analyses. However, the results of these analyses suggest that the retardation and agitation subscale scores do not yield a meaningful “total” score. The MARS is a dual subscale system for the assessment of motor symptoms in depression, and the agitation and retardation subscale scores should be applied separately.
It has been suggested that motor abnormalities are the most clinically apparent indication of neurologic dysfunction in depression.
4 The MARS may be used for the further investigation of a full complement of motor signs associated with depressive disorder. Although past instruments have included items reflecting non-interactiveness and cognitive slowing, it is likely that when motivational/cognitive and motor factors are rated simultaneously, raters' judgments of observable motor phenomena become biased. Furthermore, it is likely that these nonmotor phenomena are most reliably assessed by means of standardized task-based measures rather than clinical observation. Thus, as part of a battery of tests that separately assesses motor and motivational/cognitive functions, the MARS could also be useful for specifying the relative contribution of motor abnormalities to the “psychomotor” symptoms in depression.
ACKNOWLEDGMENTS
The authors thank Dr. Stanley Arkow, the staff of Eye 6, Dr. John Nee, and Dr. Sarah H. Lisanby for their help in the completion of this work. This study was supported by a Young Investigator Award from the National Alliance for Research in Schizophrenia and Depression (NARSAD) and, in part, by the New York State Office of Mental Hygiene.