Behavioral disturbances complicate the course of up to 60% of individuals with dementia,
1 cause considerable distress to both patients and caregivers, and often lead to nursing home placement because patients cannot be managed at home. Multiple nonpharmacologic and pharmacologic treatments may be used in an attempt to ameliorate these symptoms.
2 Neuroleptics in particular have been shown to have a moderate benefit over placebo in treating behavioral disturbances in dementia, but conventional high-potency neuroleptics such as haloperidol carry the risk of extrapyramidal side effects (EPS), including parkinsonism and tardive dyskinesia.
3–5Clinicians and researchers have been interested in the potential of new atypical neuroleptic agents for greater efficacy and/or decreased side effects in this population. Risperidone is a high-potency new-generation neuroleptic that shares with the other atypical antipsychotics (clozapine, olanzapine, quetiapine) a mechanism of combined dopamine D
2 receptor and serotonin 5-HT
2 receptor antagonism. This pharmacologic profile is postulated to mediate antipsychotic effects while having a low potential to cause extrapyramidal symptoms.
6–8 Risperidone has been reported to be effective in a broad range of neuropsychiatric syndromes, including psychosis and agitation in schizophrenia,
9 manic symptoms in mentally retarded adult patients,
10 tics in Tourette's syndrome,
11 and psychosis in Parkinson's disease.
12 It has been used to control agitation in dementia in a few naturalistic open-label reports in psychiatric treatment settings,
13–16 and a recent double-blind placebo-controlled study demonstrated modest efficacy in treating behavioral disturbances in patients with dementia residing in nursing homes.
17 The combination of high neuroleptic potency with low risk of extrapyramidal side effects
18 has led to the recent use of risperidone over haloperidol in patients seen in the Memory Disorders Unit of the Massachusetts General Hospital. This study aimed to review the clinical aspects of this therapeutic trend. We reviewed the case histories of the first 47 patients to receive risperidone in the Memory Disorders Unit to assess the hypothesis that risperidone was effective in treating target symptoms (agitation and psychosis) in the majority of patients, with minimal EPS.
METHODS
We reviewed the charts of all active patients in the outpatient Memory Disorders Unit of Massachusetts General Hospital and identified 47 patients who received risperidone from June 1994 to October 1995, of whom follow-up data were available for 41. For these patients, we obtained baseline and treatment characteristics from the patient registry and chart review. The baseline characteristics identified were the following: age, gender, dementia type, dementia severity, and duration of illness. The treatment characteristics identified were the following: indications for treatment, risperidone dose, duration of risperidone treatment, side effects, whether risperidone was stopped, and concurrent medications. The severity of dementia was assessed by the Information, Memory, and Concentration section of the Blessed Dementia Scale and by the Activities of Daily Living Scale. The Blessed Dementia Scale is a brief mental status examination scored from 0 errors to a maximum score of 37 errors.
19 The Activities of Daily Living questionnaire is completed by the caregiver.
20 It measures impairment in self-care, household activities, and employment on a scale from 0, meaning no impairment, to 100, indicating maximal dependency. The patient registry included a graded scale for assessing tone, akinesia, tremor, parkinsonian gait, armswing, and en bloc turning, assessed in detail during each clinic visit; thus, extrapyramidal signs were fully clinically documented in all cases, both prior to treatment with risperidone in all cases and at follow-up. Parkinsonism was defined by the presence of at least two of these symptoms.
Treatment response to risperidone was assessed on a three-point scale. Complete response was defined as marked improvement with complete remission of the target symptoms (score=3). Partial response was defined as moderate improvement with significant but incomplete reduction in the target symptoms (score=2). No response was defined as slight improvement (score=1) or no improvement at all (score=0) in target symptoms.
Statistical methods used involved analysis of continuous variables by unpaired t-tests and of categorical variables by chi-square tests and Fisher's exact tests. Further, a logistic regression analysis was performed on baseline characteristics to identify predictors of response and EPS.
RESULTS
Baseline Characteristics
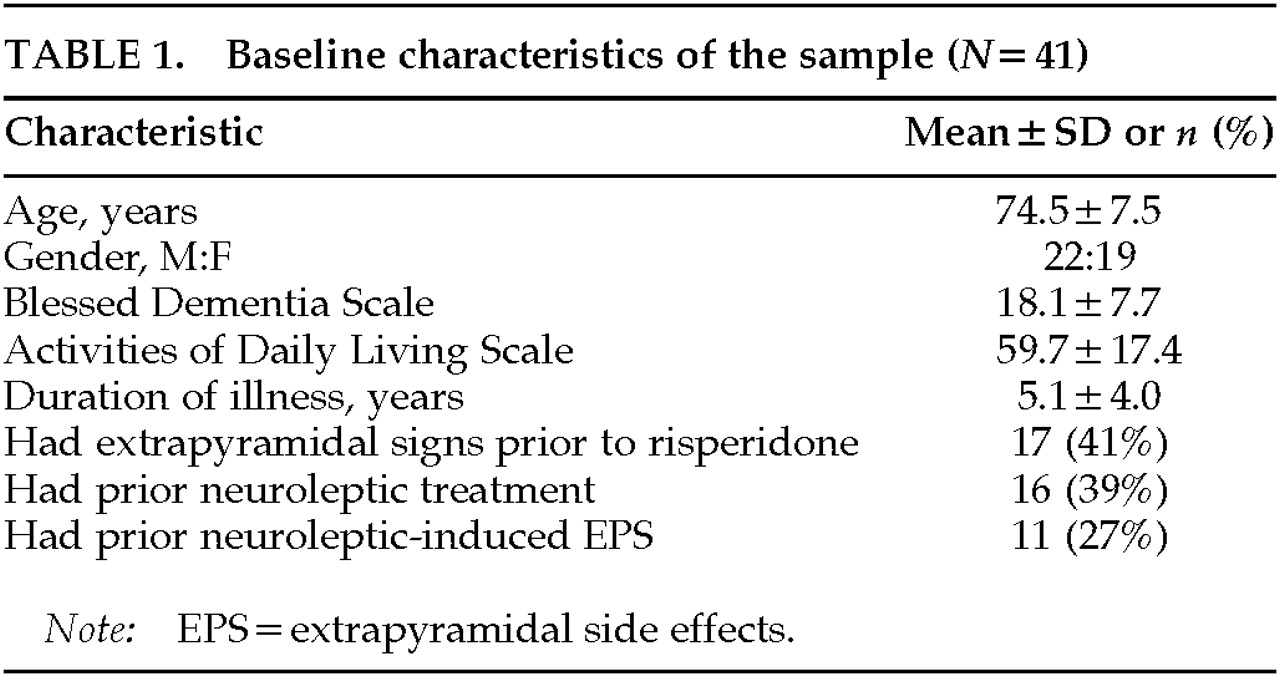
Table 1 summarizes the baseline characteristics of the sample. In the 41 treated patients with follow-up, 32 had a diagnosis of probable Alzheimer's disease (AD), 6 dementia with Lewy bodies (DLB), 1 mixed AD and Parkinson's disease (PD), 1 mixed (multi-infarct and AD) dementia, and 1 primary progressive aphasia. Many patients had long-standing disease; the mean duration was 5 years from the onset of cognitive symptoms. The mean scores on the Blessed Dementia Scale and the Activities of Daily Living Scale were in the moderately to severely impaired range.
Extrapyramidal signs were present before initiation of risperidone in 17 patients (41%). Bradykinesia and rigidity were the most common extrapyramidal signs, found in 27% and 29%, respectively; tremor was rare, being documented only in 2%. This group of patients with prior extrapyramidal signs included 4 patients who had Alzheimer's disease with mild extrapyramidal signs, 7 who had DLB or PD, and 6 who had EPS attributed to prior neuroleptic use.
Treatment Characteristics and Clinical Response
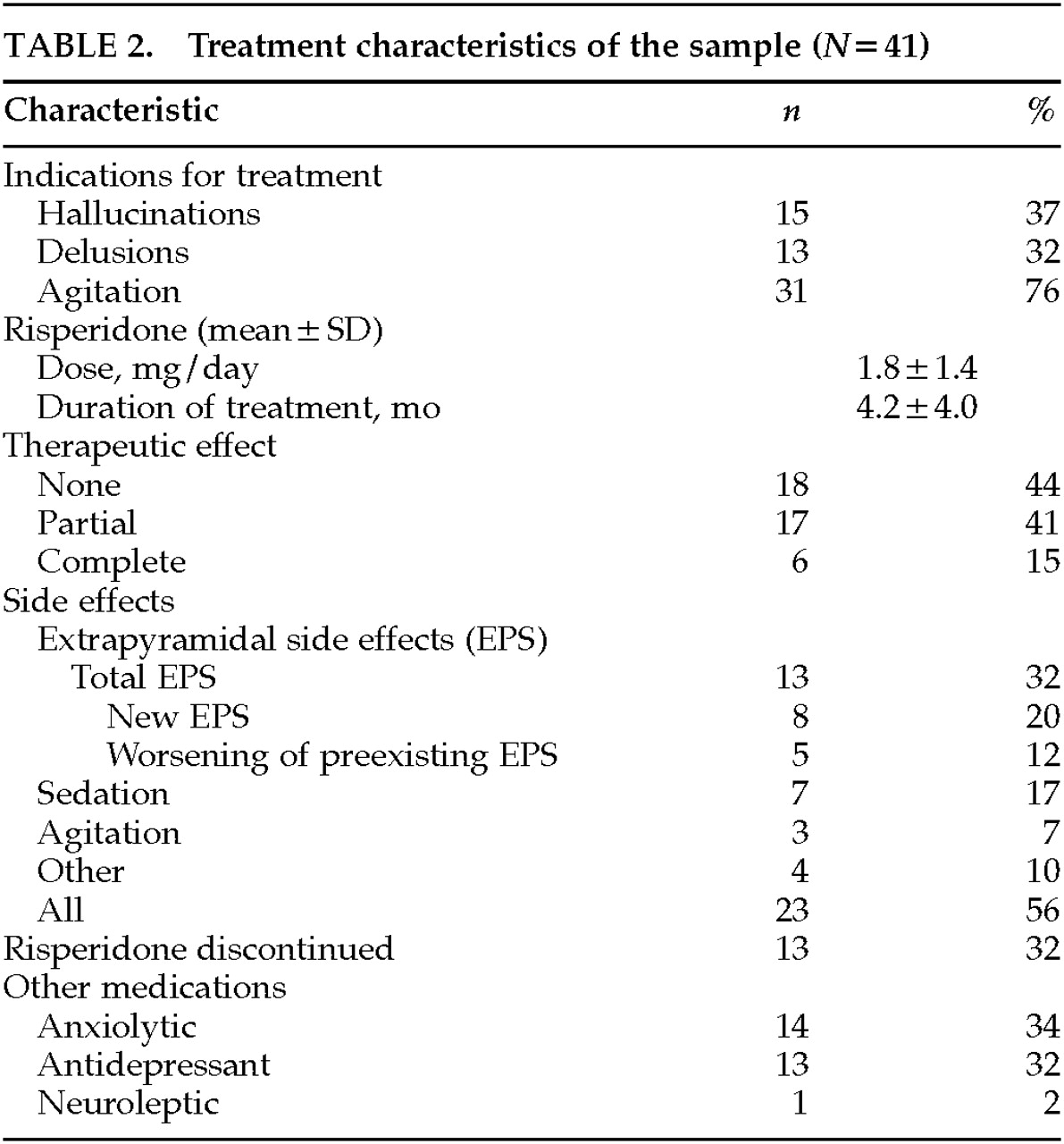
Table 2 describes the treatment characteristics of the sample. As an indication for treatment, agitation was twice as frequent as delusions or as hallucinations. Agitation broadly included aberrant motor behavior (wandering, disrobing, and generally nonviolent disruptive behavior) and physical or verbal aggression.
21 Agitation was the most common indication present during initiation of risperidone; it was found in 76% and was the sole indication in 44%.
The average dose of risperidone was 1.8±1.4 mg per day, a relatively low dose compared with the 4 to 8 mg per day used in schizophrenia. The mean duration of treatment was about 4 months. A complete response was present in 15% of the sample, a partial response in 41%, and no response in 44%. About half of the patients experienced side effects, primarily development or worsening of extrapyramidal signs in 32%, sedation in 17%, or worsening agitation in 7%. In 32% of patients, risperidone was discontinued by the prescribing physician because of side effects or lack of efficacy. Concurrent medications included anxiolytics and antidepressants, each in about one-third of patients.
Risperidone administration was linked to EPS in 13 patients: 6 patients developed new parkinsonism, and 5 others had worsening of preexisting parkinsonian signs. There were two unusual EPS. One patient developed severe cervical dystonia at a dose of 6 mg/day, and another patient developed neuroleptic malignant syndrome at a dose of 2.5 mg/day, in the setting of phenytoin 400 mg/day, thioridazine 150 mg/day, and perphenazine 4 mg/day.
Predictors of Treatment and EPS
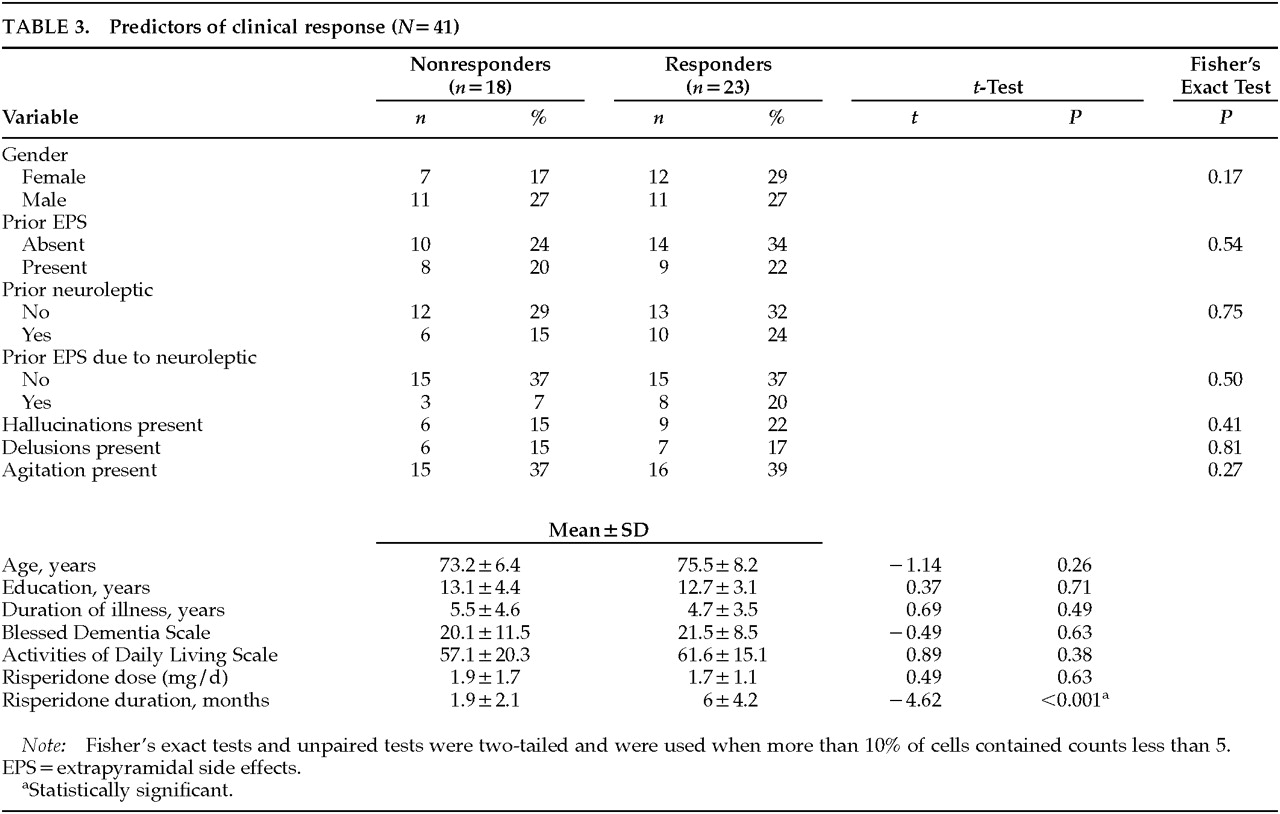
To identify predictors of treatment response, patients were divided into two groups: responders (partial or complete improvement of target symptoms,
n=23) and nonresponders (no improvement in target symptoms,
n=18), as shown in
Table 3. No statistically significant differences in baseline characteristics between the two groups were revealed by a logistic regression analysis, except for longer duration of risperidone treatment in responders. This finding may be artifactual, since nonresponders might be likely to discontinue treatment earlier than responders. In patients who had hallucinations, either alone or in combination with other behavioral symptoms, partial or complete improvement in symptoms occurred in 60%, comparable to the response in patients who had delusions or agitation.
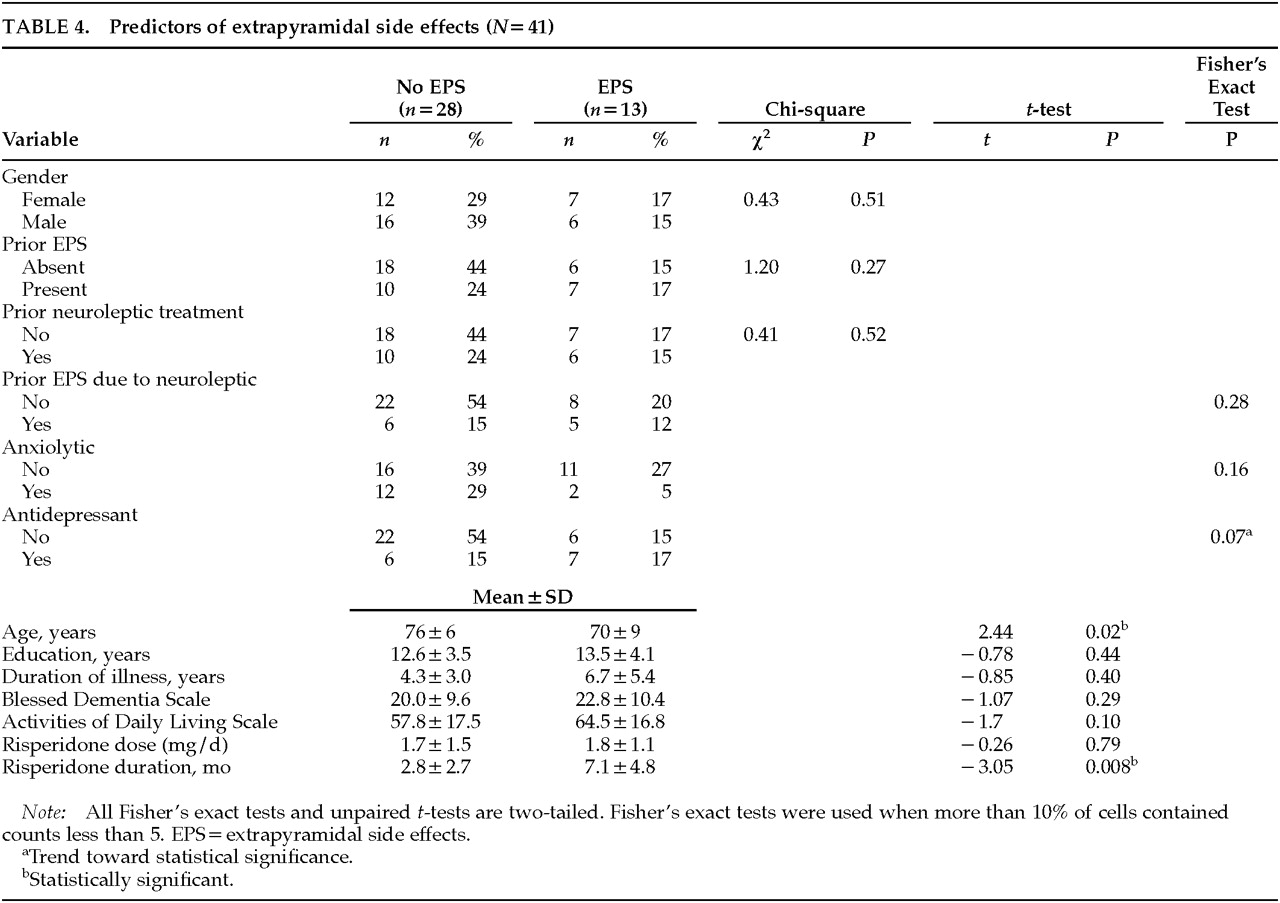
To identify predictors of developing EPS, we examined risk factors in patients who developed EPS with risperidone (
n=13) compared with those who did not (
n=28), as shown in
Table 4. Patients who developed EPS tended to be younger than those who did not (70±9 years vs. 76±6 years,
P=0.02). There was a trend toward coadministration of antidepressant medications: 54% of patients who developed EPS were on antidepressants—2 on trazodone, 1 on selegiline, 1 on both trazodone and selegiline, 2 on selective serotonin reuptake inhibitors (SSRIs; fluoxetine and sertraline), and 1 on a serotonergic tricyclic antidepressant (clomipramine)—compared with 21% of those without EPS (
P=0.07). More significantly, the duration of risperidone treatment in patients who developed EPS was more than twice the duration in patients who did not develop EPS (7.1±4.8 months vs. 2.8±2.7 months,
P=0.008). Duration of dementia, Blessed Dementia scores, and Activities of Daily Living scores were all worse in the group that developed EPS, but these differences did not reach statistical significance.
DISCUSSION
The results of this study indicate that risperidone suppressed the target symptoms of agitation and psychosis completely (15%) or partially (41%) in outpatients with dementia. Risperidone was effective in low doses, and it seemed as effective in agitation per se as in overt psychosis. Risperidone induced new EPS or worsened preexisting extrapyramidal signs in 32% of treated patients. The main predictor of EPS was longer duration of treatment.
These results agree with available naturalistic studies
14 and the recent double-blind study
17 regarding the effectiveness of risperidone in the treatment of agitation or psychosis in dementia. However, the present report, unlike the double-blind study, involves outpatients with dementia and suggests that risperidone may be helpful outside of the nursing home setting as well. The low dosages required (mean 1.8 mg/day) are consistent with those of other studies in dementia. Dosing was usually initiated at 0.5–1.0 mg/day and increased in increments of 0.5–1.0 mg weekly as needed for effect. In schizophrenia, higher doses are generally used (4–6 mg/day). Thus risperidone, like its parent compound haloperidol, appears to have broad anti-agitation effects. Its safety in patients with delirium and other serious medical conditions remains to be established.
The patient population studied is more liable to develop EPS because of increased age, past neuroleptic use, and inclusion of patients with suspected Parkinson's disease and dementia with Lewy bodies. Risperidone was not free of extrapyramidal side effects in this group, and it was associated with 1 case each of cervical dystonia and neuroleptic malignant syndrome (NMS) in relatively higher dosages or with polypharmacy. Dystonia can occur with any neuroleptic, especially at high doses, and NMS has been reported with atypical neuroleptic agents like risperidone and clozapine, alone and in cotherapy with typical neuroleptic agents.
22–27 In general, the finding that about one-third of patients with dementia developed new or worsening EPS on risperidone is comparable to the EPS prevalence of risperidone treatment in schizophrenia. Although the clinical trial data in schizophrenia have reported low rates of EPS (12% with risperidone compared with 26% with haloperidol in a representative study),
28 community-based naturalistic studies in general psychiatric samples have reported higher EPS rates (22% for risperidone vs. 55% for conventional neuroleptics in one study
29 and 48% for risperidone vs. 49% haloperidol in another study
30). The higher rate of risperidone-related EPS in naturalistic studies may reflect preexisting EPS, other concomitant medications, age, gender, and comorbid medical, psychiatric, and neurological illnesses.
The main treatment characteristic associated with EPS was
longer duration of risperidone treatment—on average, about 7 months in those who developed EPS versus about 3 months in those without EPS. Parkinsonism induced by traditional neuroleptic agents typically occurs within 3 months of treatment initiation or dosage increase,
31 although elderly individuals on stable doses remain at risk.
32 Finding
younger age a risk factor for EPS was a surprise, since old age is generally believed to be a risk for neuroleptic-induced EPS, including tardive dyskinesia, in primary psychiatric populations.
33 Our data also show a statistical trend for an association between
concomitant antidepressant use and EPS. Most of these patients who developed EPS on antidepressants and risperidone were taking serotonergic antidepressant agents (trazodone, clomipramine, fluoxetine, sertraline). SSRIs have been associated with movement disorders, as monotherapy or in combination with other agents. Akathisia is the most common extrapyramidal side effect related to SSRIs, although dystonia, parkinsonism, and tardive dyskinesia have also been described.
34–36 These effects may be mediated by pharmacodynamic interactions between serotonergic and dopaminergic neurotransmitter systems
36 or by pharmacokinetic interactions, with SSRIs inhibiting hepatic cytochrome P450IID6 enzyme metabolism of risperidone and possibly displacing risperidone from plasma protein binding.
37 Further studies would be required to replicate our initial observations.
These results should be interpreted in the context of several limitations: First, data were collected retrospectively on the basis of chart review; prospective studies are needed to confirm or refute these pilot findings. Second, some of the significant statistical findings are liable to type I multiple comparison error and thus may represent false positive findings; no corrections for multiple comparisons were performed because this study was conceived as a pilot study providing initial descriptive data for the generation of hypotheses that could be tested in future, prospective studies. Conversely, some of the nonsignificant findings are liable to type II error and may represent false negative results due to lack of statistical power. Third, the risk of EPS may be overestimated, since extrapyramidal signs are known to occur in both AD and DLB.
38,39 Nonetheless, these results are still useful in suggesting how effective risperidone might be in the real-world clinical setting. Despite acknowledged limitations, these pilot results should help in the design of future controlled studies to determine optimal treatment of demented patients with agitation and psychosis.