Many neuropeptides, through direct cerebrospinal fluid (CSF) measurement, have been implicated in the pathogenesis of affective illness.
1–3 In the case of thyrotropin-releasing hormone (TRH), two clinical studies
4,5 have reported elevated levels of CSF TRH in patients with acute depression, while a third study
6 was negative. Of particular interest is the question of whether this TRH elevation or “hypersecretion” should be conceptualized as primary pathology—i.e., related to the mood disorder itself—or as a compensatory response to maintain homeostasis with potential therapeutic benefit.
1In addition to its role in the hypothalamic-pituitary-thyroid axis (HPT), many preclinical studies implicate TRH in a number of extra-HPT neuromodulatory processes. These processes have been compartmentalized into TRH “subsystems,” including limbic system-amygdala-hippocampus,
7–14 preoptic anterior hypothalamus–suprachiasmatic nucleus,
15 and brainstem–dorsal raphe.
16Neuroendocrine circadian rhythms must be taken into account when measuring TRH secretion into cerebrospinal fluid. Basic studies in rodents have identified diurnal rhythms in TRH content in both hypothalamic and extrahypothalamic locations.
17 Specific rhythms in brainstem
18 and cortex
19 TRH levels have been identified that show periods separate and distinct from rhythms expressed in the hypothalamus. Clinical studies involving TRH measurements in human CSF have not addressed the question of circadian fluctuations in peptide concentrations. A single study in rhesus monkey,
20 however, has examined this issue. TRH levels measured in the monkey were similar to those measured in humans, showing afternoon increases of approximately 33% of baseline levels. CSF TRH levels in the rhesus monkey were at baseline levels between 0800 and 1000 h. Although direct extrapolation cannot be made to humans, the observed nyctohemeral rhythm of human TSH secretion
3 would be consistent with the TRH pattern observed in monkey. Given that lumbar punctures were performed at 0800–0900 h in the present study, TRH levels were presumably at or near baseline.
TRH administration in a number of animal models of depression has been reported to exert antidepressant properties,
2,21 and in many clinical studies, as recently reviewed by Callahan et al.,
22 TRH has shown antidepressant efficacy. In light of the postulated role of TRH as an endogenous antidepressant, this investigation was conducted to further assess the CSF levels of TRH in a refractory mood-disorder population.
METHODS
Fifty-six depressed inpatients (35 female, 21 male; 28 bipolar, 28 unipolar; average age 39.8±12.7 years) were studied in the Biological Psychiatry Branch, National Institute of Mental Health (NIMH). Their mood disorder was confirmed with the Structured Clinical Interview for DSM-III-R or DSM-IV shortly after hospital admission. They were otherwise healthy; specifically, there was no history of endocrinological illness, thyroid supplementation, or current alcohol use. Eleven of the patients had exposure to lithium in the 3 months prior to the lumbar puncture. Thirty-four medication-free control subjects (12 female, 22 male) with an average age of 32.5±10 years were used as a comparison group. Written informed consent was obtained after the lumbar puncture procedure had been explained to the patients and the control subjects.
Patients were in a double-blind, medication-free period for at least 2 weeks (mean=31.5±2.54 days). All of the patients remained symptomatic of their illness during the placebo washout. Both patients and control subjects adhered to a monoamine-restricted, caffeine-limited diet for the 2 weeks preceding the lumbar puncture. CSF was collected according to a standardized method that included an overnight bed rest and fast, with the procedure completed in a lateral decubitus position between 0800 and 0900 h (TRH levels presumably at or near baseline). To evaluate the effect of medications on mood and CSF TRH, a second lumbar puncture for patients was often obtained after 6 weeks of a blinded study medication (carbamazepine, nimodipine, lamotrigine, gabapentin, venlafaxine, or bupropion). Mood at the time of lumbar puncture was assessed by using a modified version (28 items) of the Hamilton Rating Scale for Depression (Ham-D).
23An aliquot of the 26th–27th cc of CSF fluid removed was placed in a –70°C freezer until the time of TRH radioimmunoassay. Human CSF aliquots of 100 μl were assayed in duplicate according to the method of Bassiri and Utiger.
24 Assay sensitivity was 0.49 pg per tube, and the inhibitory concentration (IC
50) was 30 pg. The interassay variation was 2.89%. All immunoassays were performed blind with regard to mood state and diagnosis.
To review the patient's past medical history, we used the retrospective life chart method NIMH-LCM™.
25 The LCM™ allows for systematic quantification of a number of retrospective course-of-illness variables, including duration of illness, age at first symptoms, and number of prior episodes and hospitalizations. The mean length of acute episode and the duration of illness for this patient group were 10.6±2.7 weeks and 24.55±2.26 years, respectively.
In order to test previous reports of elevated CSF TRH, we used a two-factor analysis of variance (ANOVA) with post hoc t-tests, where indicated, to assess TRH differences by gender and diagnosis. Paired t-tests were used to assess differences in CSF TRH between conditions: on/off medications and “ill”/“well” (i.e., Ham-D score ≥18/<18). Finally, retrospective life chart measures were analyzed by Pearson correlation. Means plus or minus standard deviations are reported.
RESULTS
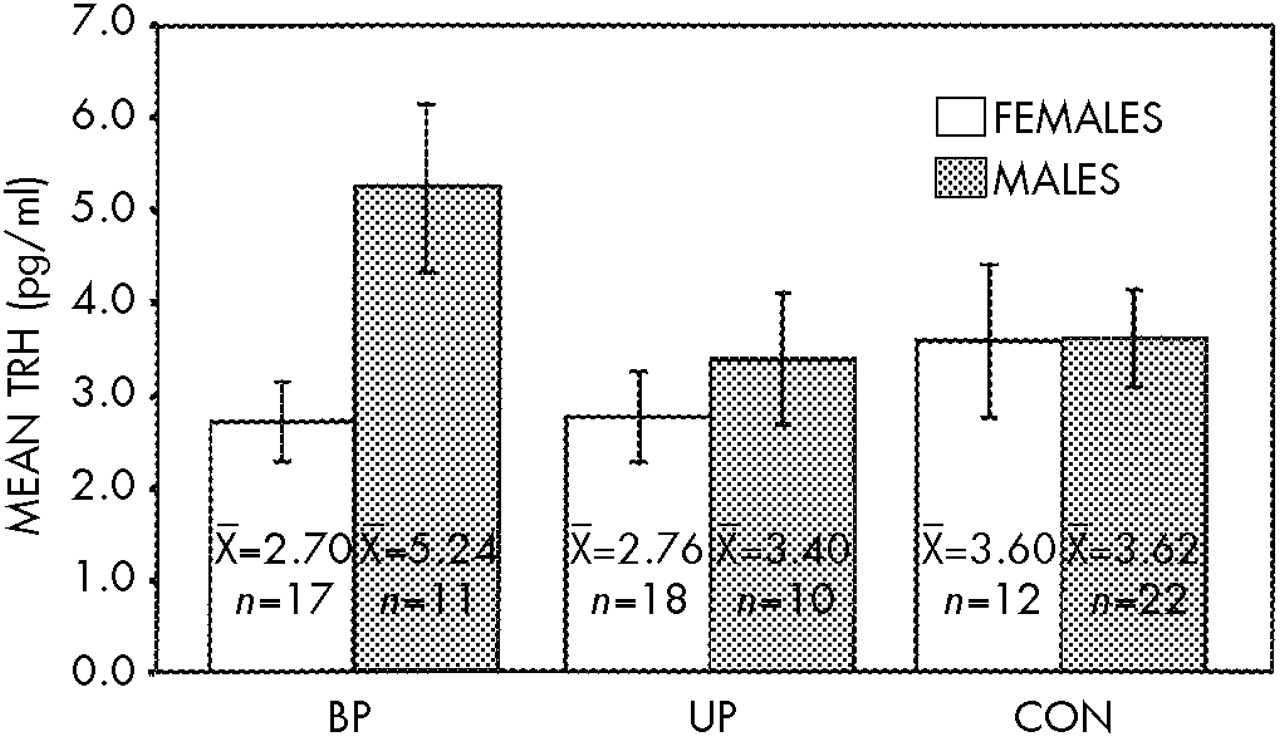
As presented in
Figure 1, two-way ANOVA found no difference between CSF TRH in patients (as a group or by diagnostic subtype) and control subjects (bipolar, mean=3.70 pg/ml; unipolar, mean=2.99 pg/ml; control, mean=3.61 pg/ml;
n=90,
F=0.91, df=2,84,
P=0.41). There was, however, a CSF TRH gender difference (females, 2.95 pg/ml; males, 3.98 pg/ml;
n=90,
F=4.11, df=1,84,
P<0.05). A post hoc
t-test revealed the greatest gender difference for the bipolar group (
t=2.52,
P=0.02) compared with the unipolar (
t=0.74,
P=0.50) and control groups (
t=0.024,
P=0.98). There was no significant CSF TRH difference between patients who had exposure to lithium in the 3 months prior to the lumbar puncture (
n=11, mean=2.93 pg/ml) and patients who did not have lithium exposure within the 3 months (
n=44, mean=3.45 pg/ml,
t=0.652, df=53,
P=0.52).
There was no correlation between CSF TRH and age (n=90, r=0.03, P=0.78). There was a correlation between CSF TRH and height (n=71, r=0.25, P=0.038) that was not significant when broken down by gender (n=38 female, r=0.17, P=0.30, n=33 male, r=0.05, P=0.78). There was no correlation between CSF TRH and length of time from lithium discontinuation (n=11, r=–0.34, P=0.31).
There was no correlation between CSF TRH and mean Ham-D score (P=0.63), length of acute depressive episode (n=42, r=–0.10, P=0.95), or duration of affective illness (n=41, r=–0.25, P=0.12). When CSF TRH was analyzed on/off lamotrigine or gabapentin (n=10), nimodipine (n=16), carbamazepine (n=15), or the antidepressants venlafaxine or bupropion (n=13), no significant changes were noted. Similarly, there was no significant difference in CSF TRH in “ill” versus “well” state (n=20, P=0.41).
There was no relationship between CSF TRH and age at first symptoms (P=0.28), number of prior episodes (P=0.58), or hospitalizations (P=0.73). Analysis by gender, however, revealed that males tended to have a positive correlation between CSF TRH and prior episodes (n=17, r=0.50, P=0.04) and hospitalizations (n=17, r=0.42, P=0.093).
DISCUSSION
These data in a refractory mood-disorder population with prolonged course of illness (approximately 25 years) do not confirm previous reports of elevated CSF TRH in acute depression.
4–6 Furthermore, the lack of a state-dependent correlation (i.e., correlation with the Ham-D scale or “ill”/“well” state) replicates two
5,6 of the three previous studies. (Kirkegaard et al.
4 did not report severity of illness.) Additionally, our study did not find a difference in levels of CSF TRH on/off various agents, including carbamazepine, and thus does not replicate the previous report of Marangell et al.
26Notably, among these studies Banki et al.
5 had the shortest acute episode length (3 weeks) associated with CSF TRH elevation; this finding contrasts with the present study and that of Roy et al.,
6 where acute episode lengths of 2.5 months and 7.8 months, respectively, were not associated with a CSF TRH elevation. In addition to the prolonged length of acute episode in the present study, the lack of CSF TRH elevation could be related to a longer duration of illness (24.5 years) when compared with the only previously documented duration of illness (7 years).
5 Kirkegaard et al.
4 did not report duration of acute episode or course of illness. Chronicity of illness could thus possibly contribute to a neuroendocrine “burnout,” which could theoretically erase a neuroendocrine perturbation, pathologic or compensatory, that was present earlier in an acute episode or course of illness. Although this hypothesis is quite speculative, it is supported by animal studies that show central TRH receptor downregulation in various brain regions following chronic exposure to TRH.
27 The positive correlation in our study between CSF TRH and prior episodes and hospitalizations in males, however, does not support this hypothesis.
The CSF TRH gender difference in the present study appears not to be related to age, height, or, for patients, current severity of depression, duration of acute episode, or duration of illness. This study, comprising 90 subjects (47 female), provides greater power to detect a gender difference in comparison to the three previous studies,
4–6 which comprised 46 patients (35 identified as female). This gender difference is similar to that found in the study by Fossey et al.,
28 where male subjects had higher CSF TRH levels than female subjects (2.30±0.95 pg/ml vs. 1.69±0.80 pg/ml;
t=2.61, df=54,
P<0.02). It may be that the gender difference reported in the present study should be conceptualized as a relative increase in CSF TRH levels in males. Thus, the positive correlation between number of prior episodes and CSF TRH in affectively ill males could suggest a residual-state measure of prior episodes.
Alternatively, the gender difference could be conceptualized as a relative decrease in CSF TRH levels in women. This difference does have important implications, given epidemiological studies that indicate a higher lifetime prevalence rate of major depression in women.
29 Additionally, as reviewed by Leibenluft,
30 females with bipolar disorder have a greater propensity than bipolar males for rapid cycling, perhaps related to a “labile switch mechanism.” With the greatest gender difference appearing in the bipolar group, it is interesting to speculate whether the bipolar female depressed population has a TRH deficit that, when corrected, is associated with mood stabilization or switch “quiescence.” No formal test of this hypothesis has appeared in the literature.
The clinical literature for TRH as a somatic treatment for depression is extensive and complex.
22 These complexities stem from evolving diagnostic criteria over 20 years of clinical experience, as well as differences in study design (case reports vs. open and double-blind studies), study duration, route of TRH administration (intravenous, subcutaneous, intrathecal), and criteria for response. Recently, Marangell et al.
31 reported a significant reduction in depressive symptoms with intrathecal TRH (500 μg) in patients with severe refractory depression; further analysis of baseline CSF TRH (
n=11) did not show a difference between intrathecal responders and nonresponders (Post et al., 1998, unpublished observations).
These data, although not consistent with previous reports of elevated CSF TRH in depression, do suggest important gender differences. The role of elevated levels of CSF TRH in affectively ill men or the role of decreased levels of CSF TRH in affectively ill women remains to be investigated but could be of pathophysiological relevance. Further study should be encouraged to clarify whether this gender difference has any potential implications for treatment of refractory mood disorder.