In 1995, an estimated 1.5 million Americans used cocaine, and the number who used cocaine weekly was around a half million. The social and medical consequences of this epidemic of cocaine use make it a significant public health problem. Although there are numerous studies on the long-term effects of alcohol on neurocognitive functioning, there are few reports on the persistent neurobehavioral effects of cocaine abuse. Unfortunately, most studies that do exist are preliminary reports, and the methodology is problematic. For example, freebase cocaine users showed difficulties with visuomotor tracking, cognitive flexibility, and speed of information processing (executive functioning) after 10 days of abstinence.
1 However, these results must be interpreted with caution because no control group was included. Ardila et al.
2 also did not include a control group for comparison when describing deficits in short-term verbal memory and attention in cocaine users. Furthermore, they used published norms to define an abnormality. Design flaws have also been noted in studies using control groups. For instance, in a study including control subjects, the abstinent cocaine group had deficits in short-term memory; however, length of abstinence was not reported.
3 When length of abstinence was estimated to be greater than 4 weeks in other studies, deficits persisted in short-term memory,
4,5 concentration, nonverbal problem solving, and abstraction ability.
5 When urine drug screens were used to verify recent cocaine use and length of abstinence, deficits were found in memory, visuospatial abilities, and concentration after 2 weeks of abstinence.
6 It is important to control for length of abstinence to facilitate comparisons among studies.
Overall, findings on the neurocognitive effects of chronic cocaine abuse are equivocal. Most studies report difficulties with response speed, visual and verbal learning and memory, and executive functions.
2,3,6–12 Others, in contrast, report minimal effects despite decreased cerebral blood flow;
13,14 however, these studies failed to present detailed results of the specific tests used. On the other hand, Manschreck et al.
3 found performance enhancements on some tests (e.g., finger tapping, visuospatial memory and learning) with chronic cocaine use. These discrepant results can probably be attributed to differences in the amount and/or duration of cocaine use, differences in the route of administration, the length of abstinence when tested, the sensitivity of the neuropsychological tests given, lack of a control group, small sample size, and/or poor control for age, premorbid level of intelligence, or concurrent use of alcohol.
METHODS
Subject Selection
All participants were recruited by Nova Research Corporation. Nova uses newspaper advertisements to recruit for participants for all NIH National Institute on Drug Abuse Intramural Research Program (NIDA-IRP) protocols. Subject selection was based on drug use history, which was obtained from all participants by using a structured interview, the Drug Use Survey Questionnaire (DUSQ),
16 the Addiction Severity Index (ASI),
17 and the Diagnostic Interview Schedule (DIS).
18 The DIS was also used to diagnose past or current history of psychiatric illness (e.g., major affective disorder, schizophrenia, alcohol dependence or abuse, drug dependence or abuse).
The cocaine group consisted of individuals who claimed cocaine as their drug of choice, used cocaine by any route for at least 1 year, administered cocaine at least four times a month, and had a urine toxicology screen that was positive for cocaine metabolites at the time of admission into the study. This positive screen indicated cocaine use during the past 24 to 48 hours and ensured that all participants were abstinent for a uniform period. Participants were still included if dependent on caffeine or tobacco. Participants were excluded if they met DSM-III criteria from the DIS for current or past dependence on any other psychoactive substance other than cocaine, including alcohol, or if their urine toxicology screen was positive for substances other than cocaine and its metabolites. Additional exclusion criteria are detailed below.
Participants were excluded from the control group if they met DSM-III criteria for past or current dependence on or abuse of any substance except nicotine. Volunteers were also excluded if they self-reported a history of drug use other than alcohol, if they drank more than four times in the past 30 days, or if their urine toxicology screen was positive for any illicit drugs, including marijuana.
Volunteers were excluded from both participant groups if they had a past or current psychiatric disorder by DSM-III criteria from the DIS. Therefore, participants with disorders such as generalized anxiety disorder, posttraumatic stress disorder, and major depressive disorder were excluded. Volunteers were also excluded if they reported a past or current history of neurologic illness (e.g., head trauma resulting in loss of consciousness, seizure disorder, stroke), had an abnormal neurologic examination, had a diagnosis of alcohol dependence or abuse, or were pregnant.
Data Collection
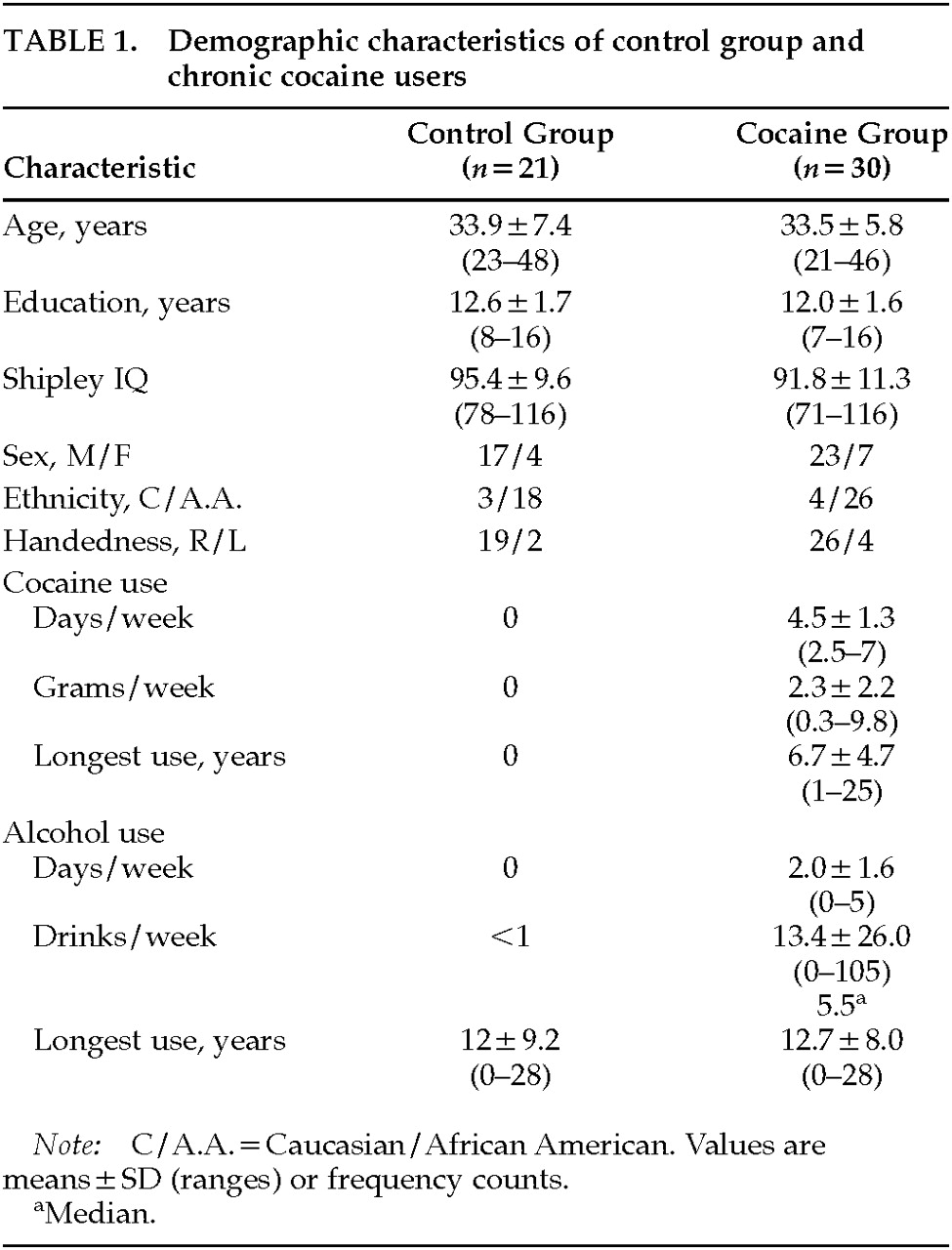
At the initial visit to the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP, the cocaine group received a thorough medical evaluation including a urine toxicology screen and a pregnancy test for women. Participants in the cocaine group were admitted to the CIRU for 30 days. No treatment or medications were given over the 30-day stay. The non–drug-using participants were tested as outpatients. All participants were given neurological, neuropsychological, and psychiatric evaluations. For both groups, drug use history was obtained by a trained interviewer using the ASI and DUSQ. Participants were asked to estimate the number of days a drug was used in the last 14 days, the number of days a drug was used in a month, the number of years used (lifetime use), age at first use, and duration of longest use for all drugs used, including alcohol. Estimates were made of overall average amount and frequency of cocaine use (grams per week). Grams per week were estimated from participants' reports of how much money was spent each week ($100/gram, 50% purity; Drug Enforcement Agency reports for the Baltimore area). All participants gave written informed consent, and this protocol was approved by the Institutional Review Board. All participants were compensated for their time.
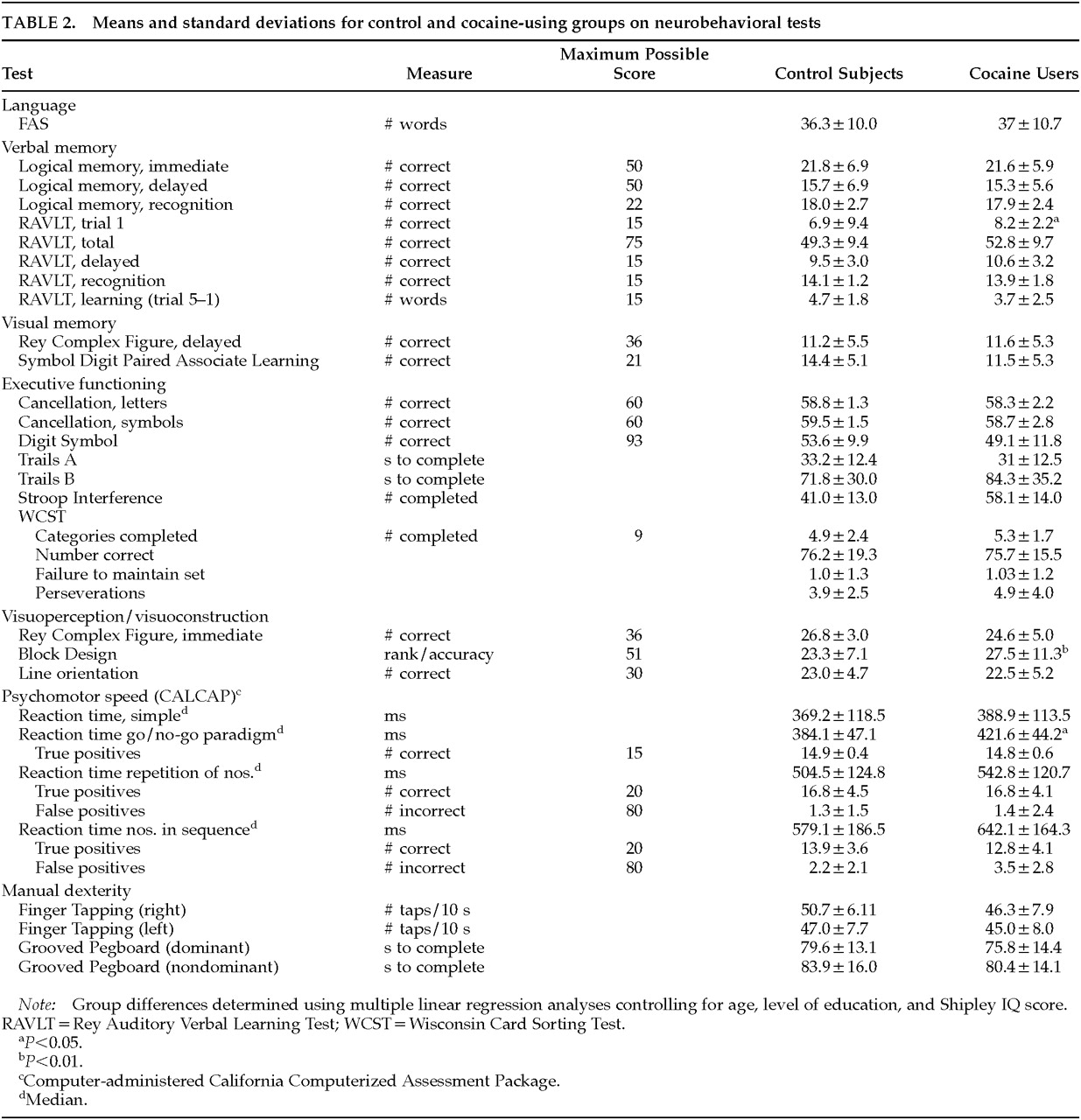
The neurobehavioral test battery was administered by a trained psychometrician under the supervision of a neuropsychologist (K.B.). The neurobehavioral battery consisted of tests that assess a variety of cognitive domains. General intelligence was estimated by using the Shipley Institute of Living Scale.
19 The Shipley estimated IQ correlates with the WAIS-R (referenced below) Full Scale IQ (
r=0.79). Measures of IQ are believed to be good estimates of native intellectual abilities (premorbid intelligence) and are resistant to the effects of brain injury. Language skills were assessed by using Controlled Oral Verbal Fluency.
20 Verbal memory was assessed by the Logical Memory from the Wechsler Memory Scales–Revised (WMS-R)
21 and the Rey Auditory Verbal Learning Test (RAVLT),
22 and visual memory was assessed by the Rey-Osterrieth Complex Figure
23 and the Symbol Digit Paired Associate Learning Test.
24 Executive functioning (attention/planning/mental flexibility) was assessed by the Verbal and Non-Verbal Cancellation Test
25 for both randomly placed letters and symbols, Digit Symbol Substitution from the Wechsler Adult Intelligence Scales–Revised (WAIS-R),
26 Trails A, Trails B,
27 Stroop,
28 and the Wisconsin Card Sorting Test (WCST).
29 The Rey Complex Figure (copy), Block Design (WAIS-R),
26 and Judgment of Line Orientation
30 assessed visuoperception/visuoconstruction. The computer-administered California Computerized Assessment Package (CALCAP)
31 was used to assess both simple and choice reaction times (psychomotor speed). Manual dexterity was assessed by using Finger Tapping
27 and Grooved Pegboard.
32 Participants were tested on the 28th or 29th day after admission to the research unit. This eliminated any acute drug effects and possible confounding from the physical or psychological symptoms associated with drug withdrawal. All testing was performed in the morning to reduce diurnal fluctuations in performance. The examiner was blind to the intensity or duration of drug use. Urine screens were performed randomly throughout the 30-day inpatient stay in order to ensure drug abstinence.
For cocaine use, participants estimated frequency of drug use for the past 30 days; that is, days per week (frequency), grams per week (intensity), and duration of longest use (duration). The cross-product of grams per week and duration of longest use was calculated to estimate total cocaine use (grams per week×years). This total use estimate reflects a combination of intensity and duration. When there was a discrepancy between the Addiction Severity Index and the Drug Use Survey Questionnaire, an average was taken. For alcohol consumption, estimates were made of days per week, duration of longest use, and number of drinks per week. The drinks per week estimate was based on the number of containers of alcohol consumed where a container was equal to one glass of wine, one shot, or 12 ounces of beer. Total alcohol use (drinks per week×years) was estimated by forming the cross-product of numbers of drinks per week and duration of longest use.
Statistical Analyses
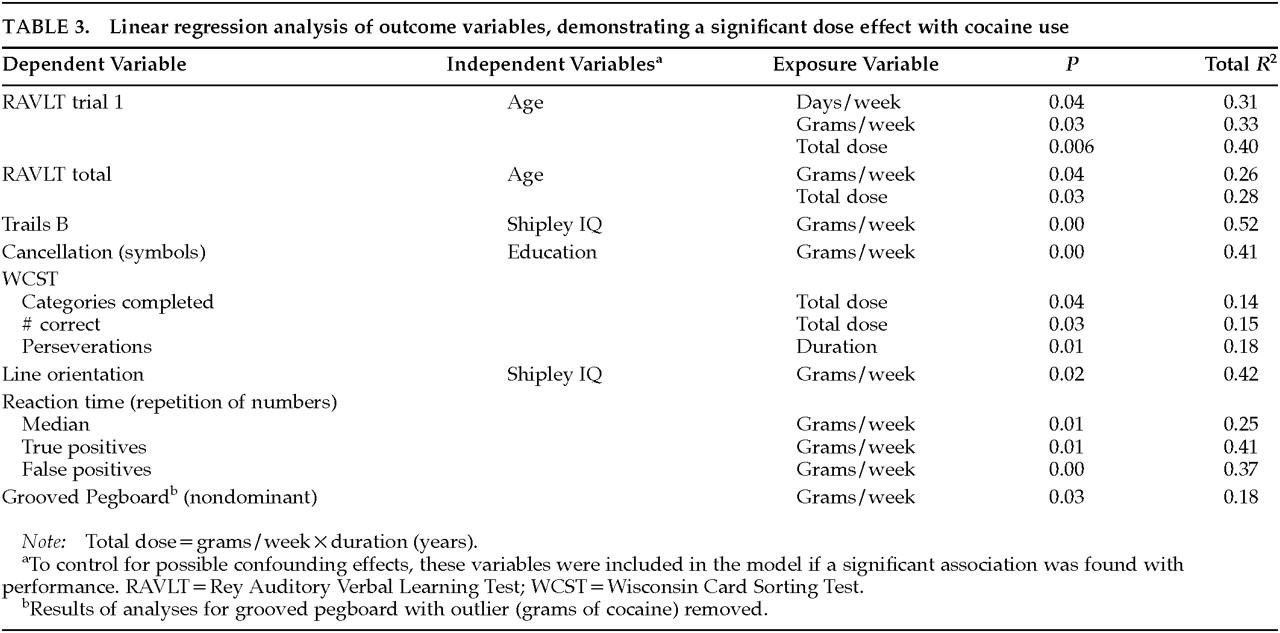
From a sample of 45 non–drug-using control subjects, we were able to individually match 21 control subjects to the chronic cocaine users on age, education, and Shipley IQ score. Although the groups were matched on demographic variables, previous studies indicate that each of these variables can significantly influence performance on neurobehavioral tests. Therefore, these possible confounding variables were included in the regression models to control for any individual differences between groups and within groups. Accordingly, exploratory analyses included age, Shipley estimated IQ, level of education, alcohol use, and group (control or cocaine user) in the models. An independent variable was retained in the model if significantly associated (
P<0.05) with the neurobehavioral outcome variable. Interaction terms (i.e., Shipley IQ×group or dose) were also examined. Every variable used in the analysis was inspected for outliers (greater than 3 standard deviations from the group mean). When an outlier was present, the data were analyzed with and without the outlier. A separate multiple regression analysis was performed for each of the neurobehavioral tests to determine group differences. Because neurotoxic agents such as lead and solvents affect the nervous system predominantly at higher doses,
15,33,34 it was desirable to establish a dose-related relationship between amount and duration of cocaine use and possible neurobehavioral decrements. Therefore, a second set of analyses was performed, substituting the self-reported drug use variables (grams per week, times per week, duration) for group within the models. All analyses were performed with the SAS statistical software program.
DISCUSSION
This study determined that chronic heavy cocaine users have persistent decrements in neurobehavioral performance despite a 4-week abstinence. A dose-related effect was found; thus, the more grams per week of cocaine used, the lower the performance. In addition, the intensity of cocaine use (grams per week) was more strongly related to alterations in neurobehavioral performance than frequency (times per week) or duration (years) of cocaine use. Few effects were found comparing a non–drug-using control group to the cocaine group. This was expected, since we have found similar results in groups of methylenedioxymethamphetamine (MDMA) users
35 (unpublished data), as well as solvent
33 and lead workers.
15 However, we did not expect the control group, taken as a whole, to perform worse on some of the tests compared with the cocaine group. The reason for this difference is unclear, since the groups were well matched on age, education, and Shipley estimated IQ. One possible explanation is that the control group was tested on an outpatient basis, resulting in our inability to control their activities the night before and the morning of testing. Although we asked about alcohol use and hours of sleep the night before testing, it is possible that misinformation was given.
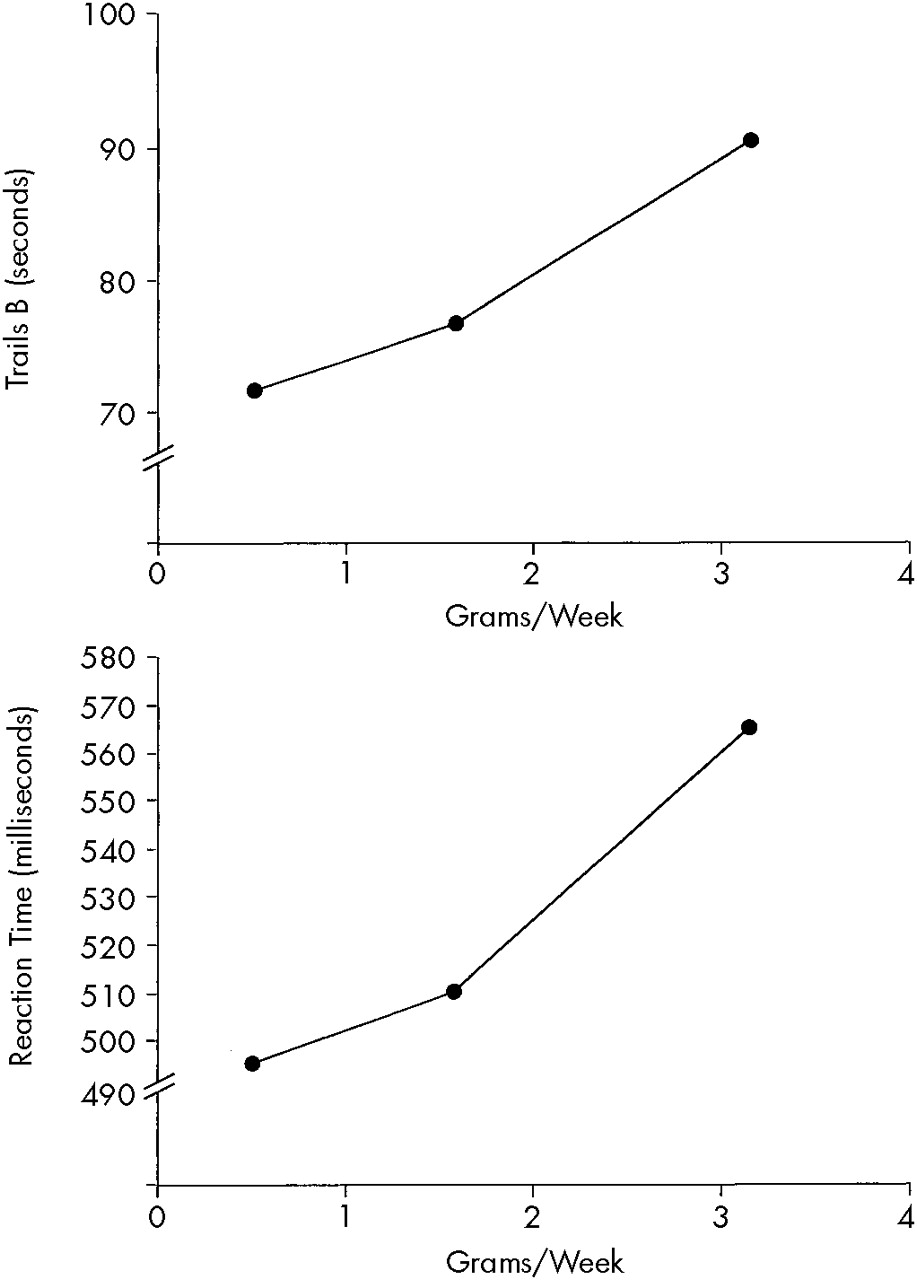
Dose-related effects were found primarily on complex tasks of higher cortical functioning involving an integration of multiple cognitive abilities (attention, planning, mental flexibility, executive functioning, psychomotor functioning). The neurobehavioral substrate associated with these behaviors is probably the prefrontal cortex. Dose-related effects were found on Trails B, the Wisconsin Card Sorting Test (WCST), and a match-to-sample reaction time test. Because of their high sensitivity, these tests generally detect decrements related to neurologic dysfunction. The Trails B and WCST tasks both involve cognitive flexibility, but the WCST also involves the ability to use feedback to monitor and change behavior. The computer-administered Repetition of Numbers Reaction Time Task requires the participant to respond when a number presented on the computer monitor is identical to the number preceding it (match-to-sample). The heaviest cocaine users showed slower median reaction times and made more errors of omission (true positive) and commission (false positive). (See
Table 4.) False-positive errors may reflect a tendency to be impulsive. It is interesting to note that lesions of the dorsolateral prefrontal cortex in primates produce decrements in performance on similar, previously learned match-to-sample tasks.
36Dose-related decrements were also found in attention and concentration. For example, heavier cocaine use was associated with lower performance on the cancellation test for randomly placed symbols, a measure of attention and concentration. In addition, adverse associations were found between cocaine use and performance on a memory test that is sensitive to attentional problems. The RAVLT consists of the presentation of a 15-word list over five trials. Trial 1 performance of the RAVLT was significantly associated with three of the four cocaine use variables. Since there were no dose-related effects on the fifth trial of the RAVLT, the association between grams of cocaine used and the total RAVLT score is probably due to the poor performance on the first trial. Poor performance on trial 1 only, with relatively intact performance on later trials, is seen in individuals who have intact memory but who become confused by stimulus overload.
37 Therefore, poor performance on RAVLT 1 and RAVLT total are probably not due to difficulty with verbal memory, but rather stimulus overload.
We acknowledge that this study had several limitations, including an inability to show causality, the use of multiple comparisons, and an inability to generalize these findings to many cocaine users because we tested a select group. These issues are discussed below. Although we believe that heavy cocaine use can potentially lead to alterations in brain functioning, we acknowledge that the dose-related decrements in neurobehavioral performance do not prove that cocaine abuse causes brain dysfunction.
An alternative explanation for our findings is that preexisting neurobehavioral differences predisposed certain individuals to use more cocaine. However, we are doubtful of this latter interpretation for a number of reasons. First, no correlation was found between Shipley IQ, an estimate of premorbid functional ability, and grams per week (
r=0.016), indicating that lower functioning individuals do not use more cocaine. Second, despite making multiple comparisons, we wanted to detect small adverse effects of cocaine on neurobehavioral functioning and therefore elected to use a
P-value of 0.05 instead of a more conservative level of 0.01. Nevertheless, although multiple comparisons were made, more adverse associations were found than could be accounted for by chance alone. Third, the present results are consistent with those of other investigators who report decrements on similar tests of neuropsychological functioning in cocaine abusers.
7,10,11 Fourth, our results are biologically plausible because the intensity of cocaine use (grams per week) was more closely associated with decrements in performance than duration (years) of cocaine use. The stronger effect of intensity of cocaine use, as compared with duration of use, on neurobehavioral test performance might be related to the short biologic half-life of cocaine. This idea is supported by the finding that with substances with long elimination half-lives, such as lead, exposure duration is very critical for the development of adverse effects.
15 However, for substances like organic solvents that have short elimination half-lives, the average intensity of exposure is significantly associated with decrements in neurobehavioral performance.
15 The current findings with cocaine users are similar to those found in workers with exposure to organic solvents with respect to pattern of neurobehavioral performance decrements.
15 These findings suggest that chronic heavy use of cocaine may produce CNS effects via mechanisms similar to those of other chemically related neurotoxicants. Fifth, these decrements are not secondary to concomitant use of other drugs because participants were excluded if they had a current or past history of significant use of other substances, including alcohol. Although it is documented that alcohol use is associated with changes in the CNS,
38 our analyses revealed no effects of alcohol on the neurobehavioral performance of these participants. This finding can be attributed to the exclusion of heavy alcohol users from the study. In addition, the correlation between the number of drinks per week and the number of grams of cocaine used per week was
r=–0.03. This suggests that the greater the use of cocaine, the fewer the number of drinks consumed. Despite the line of evidence we have here been discussing, in humans a causal link between cocaine abuse and brain injury can be determined only with a prospective study.
It will be difficult to generalize these findings to many users of cocaine because of our strict selection criteria. For example, comorbid psychiatric disorders (i.e., anxiety disorders, major depression) are common in substance abusers; however, we excluded individuals with these disorders to control for the possible confounding effects of psychiatric disorders on neurobehavioral functioning. Many cocaine users are also heavy drinkers; these users were also excluded from our study. In addition, 29 of our 30 cocaine abusers administered cocaine by smoking; therefore, these results may be pertinent only to individuals who smoke cocaine. Lastly, it could be argued that the self-reports of cocaine use are inaccurate. Although the finding of a biologically plausible dose-response suggests that the estimates of drug use were accurate, this cannot be proven definitively.
On a practical level, the evaluation of neurocognitive strengths or weaknesses in chronic cocaine users has clinical implications. For example, even subtle changes from baseline level in executive functions may be critical in perpetuating addictive behavior. Deficits in executive functions, decision making, and impulsivity can also result in difficulty with self-monitoring and changing of inappropriate behaviors. Consequently, these deficits may be relevant to the chronic cocaine abuser's inability to discontinue self-destructive drug-seeking behavior. As suggested by effects observed in the WCST, the ability to use feedback to change incorrect current behaviors to subsequent correct behaviors appears to be negatively affected in chronic cocaine users. In addition, knowledge of specific cognitive processing deficits in chronic cocaine users would be useful for designing individualized drug treatment programs. Elucidation of strengths and weaknesses in specific cognitive domains, such as motor speed, could guide vocational rehabilitation programs to integrate these individuals into the work force.
In summary, the present study indicates that chronic, heavy cocaine abusers have persistent deficits in neurobehavioral functioning, and higher doses are associated with greater central nervous system effects. The accumulated evidence supports the view that these alterations in the central nervous system could represent consequences of cocaine use.