Autism is a complex developmental disorder of brain function characterized by social and communication deficits and stereotyped-repetitive behaviors. Neuroimaging studies in autistic individuals have examined the size of the corpus callosum (CC), cerebellum, total brain volume, and brainstem.
1 For the most part, these studies have not included mentally retarded autistic subjects. The rationale typically given for this is that these subjects are more difficult to scan and that their exclusion removes the possible confounding effects of mental retardation. Nevertheless, approximately 70% of autistic individuals are mentally retarded (IQ less than 70) and 40% have IQs less than 50,
2 and it cannot be assumed that findings in non–mentally retarded autistic individuals can be generalized to those with mental retardation. The wide range of IQ in autistic individuals suggests the possibility that IQ may be a marker for distinct etiologic subgroups,
2 underscoring the importance of conducting structural brain imaging studies in those autistic individuals who also have mental retardation. Thus, the primary aim of this study was to use magnetic resonance imaging (MRI) to examine recent reports of several structural brain abnormalities in mentally retarded autistic individuals.
Gaffney et al.
3 measured the callosal area on MRI in 13 autistic subjects and 35 control subjects. Although autistic individuals had a lower mean callosal area, differences did not reach statistical significance. Egaas et al.
4 carried out MRI measurements of the area of the corpus callosum in 51 autistic patients and 51 normal control subjects. They found a significantly reduced callosal size to the posterior region; however, mental age and gender were not taken into account. Whether the difference in callosal size was specific to autism or confounded by group differences in IQ could not be determined. Moreover, Egaas et al. did not adjust CC measurements for total brain size. Recent studies demonstrate that brain size in studies of autistic subjects and control subjects should be taken into account.
5Piven et al.
5 examined the midsagittal area of the CC on MRI in 35 relatively high-IQ autistic subjects and 36 IQ-comparable control subjects and adjusted their analyses for gender, IQ, and brain size. They found a significantly smaller size of the body and posterior portion of the CC in autistic subjects relative to the brain size. In the present study, we attempted to replicate and extend the findings of decreased size of the body and posterior CC by examining a sample of mentally retarded autistic individuals with more detailed CC measurements.
In 1988, Courchesne et al.
6 published an MRI study of the midsagittal cerebellar vermis in 18 autistic individuals and 12 control subjects. Autistic individuals had a significantly smaller mean area of cerebellar vermal lobules VI and VII (the neocerebellar vermis) but not of lobules I to V. This finding, however, has not been replicated consistently,
7–13 and various methodological issues were thought to explain these discrepancies.
14–18Therefore, in addition to examining CC size, in this study we also sought to reexamine for the presence of neocerebellar hypoplasia in a sample of mentally retarded autistic individuals. To our knowledge, studies involving group comparison of neocerebellar size in MRI in mentally retarded autistic individuals have not been previously reported.
METHODS
Autistic Subjects
A consecutive series of 27 subjects who attended the Autism Clinic at the Raúl Carrea Institute of Neurological Research were included in the present study. All of them had a standardized MRI scan as part of their routine clinical evaluation. Subjects with a history of a significant medical or neurological disorder (except for 2 patients with seizures) were excluded. Parents of autistic patients were interviewed with the Autism Diagnostic Interview (ADI)
19 by a clinical psychologist (V.N.) fully trained in the use of this instrument. The ADI is a reliable semistructured interview for autism, and the accompanying algorithm adequately discriminates autistic individuals from mental age–matched nonautistic comparison groups. In addition to the ADI, informants for all subjects completed the Childhood Autism Rating Scale (CARS).
20 All subjects met both DSM-IV and the ADI algorithm (based on ICD-10) criteria for autistic disorder. A pediatric neurologist carried out a structured neurological evaluation to exclude any subjects with neurological deficit, dysmorphic features, or neurocutaneous abnormalities suggestive of tuberous sclerosis or neurofibromatosis.
Comparison Group
Seventeen individuals with mental retardation who attended the Learning Disorders Clinic at our Institute were selected as a comparison group. These individuals, on the basis of having a mental age comparable to autistic subjects, were selected from a pool of 35 subjects with mental retardation who received an MRI scan as part of another ongoing imaging study. All control subjects were assessed with the ADI, and none of them met the DSM-IV/ICD-10 algorithm criteria for autistic disorder. The range of scores for this group on the CARS was 16 to 28 points. Thus, none of the control subjects had a CARS score above the cutoff for autism (30 points). None of the control subjects had a history of medical or neurological disorder (except for seizures in 2 patients).
Because of the severe degree of mental retardation of most of our sample, the Wechsler Intelligence Scales for Children could be employed only in 4 control subjects and 1 autistic subject. Twenty-five autistic subjects (93%) and 12 control subjects (71%) were assessed with the Leiter International Performance Scale.
21 Finally, 1 autistic and 1 control subject (both over 18 years of age) were assessed with the Raven's Progressive Matrices.
22 Because of the limited verbal ability in most subjects and the availability of a nonverbal IQ estimate in all our subjects, we used nonverbal IQ as a parameter for assessing comparability on IQ of our cases and control subjects.
MRI Protocol and Data Processing
Written informed consent was obtained from parents of all patients and control subjects, as well as from subjects over 17 years of age who could provide written consent. In order to complete the MRI protocol, 18 of 27 autistic subjects (67%) and 10 of 17 control subjects (59%) required anesthesia. The protocol for this study (including the use of anesthesia) was approved by the Institutional Review Board (IRB) of the Raúl Carrea Institute of Neurological Research. MRI scans were carried out by using a 1.5-tesla General Electric Signa Horizon Scanner. Slices were acquired in the sagittal plane by using a T
1-weighted sequence with the following parameters: 5-mm-thick slices, gap=2 mm, TR=480 ms, TE=14 ms, NEX=2, FOV=24×
24 cm, and matrix=256×224. Hard copies of brain slices were photographed and digitized onto a computer by using IMAGE software (National Institutes of Health, Bethesda, MD). Area measurements were carried out on a Quadra 700 computer (Apple Computers, Cupertino, CA) and analyzed by using the Digital Imaging Processing System software (Hayden Image Processing Group, Madison, WI). Measurements were carried out on the sagittal image containing the most clearly defined view of the aqueduct of Sylvius, as was done in previous MRI studies of the midsagittal cerebellum.
6 Intrarater and interrater reliability for choosing the best slice for measurement was high (
r=0.98 and
r=0.95, respectively). Blind to group membership, a neuroradiologist carried out the following measurements:
Corpus Callosum (CC):
Following the procedure of Witelson,
23 the maximal length of the CC was taken as the line joining the most anterior and posterior points of the CC. This line was used to divide the CC into anterior and posterior halves; anterior, middle, and posterior thirds; and the posterior one-fifth region. Thus, the following seven regions were measured: rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium.
To adjust for brain size, we measured intracranial area from the midsagittal MRI sections by tracing an anterior–posterior line following the inferior border of the cerebral cortex (bordering along the straight sinus) to a point corresponding to the cerebellum. A straight line was then drawn from that point to the optic chiasm. This line was extended anteriorly and superiorly along the border of the inner table. The relative CC area was calculated as [the area of the CC divided by the midsagittal intracranial area] times 1,000. Blind intrarater and interrater reliability (carried out with a technician experienced in MRI measurements) for all measurements was high (intraclass correlation>0.98).
Cerebellum:
Area measurements of the cerebellum were made following the procedure described by Courchesne et al.
6 Thus, the anterior vermis included vermal lobules I to V, the superior posterior vermis included vermal lobules VI and VII, and the inferior and posterior vermis included lobules VIII to X. Relative cerebellar area was computed by dividing the relevant area (I–V, VI–VII, VIII–X) by the midsagittal intracranial area and multiplying the result by 1,000 as described above. Blind intrarater and interrater reliability for all measurements was high (intraclass correlation>0.95).
Statistical Analysis
Statistical analysis was carried out by using means and standard derivations, multivariate analysis of variance (MANOVA), and post hoc planned comparisons. Frequency distributions were calculated with chi-square tests and a Yates' correction for expected cell sizes less than 5. All P-values are two-tailed.
RESULTS
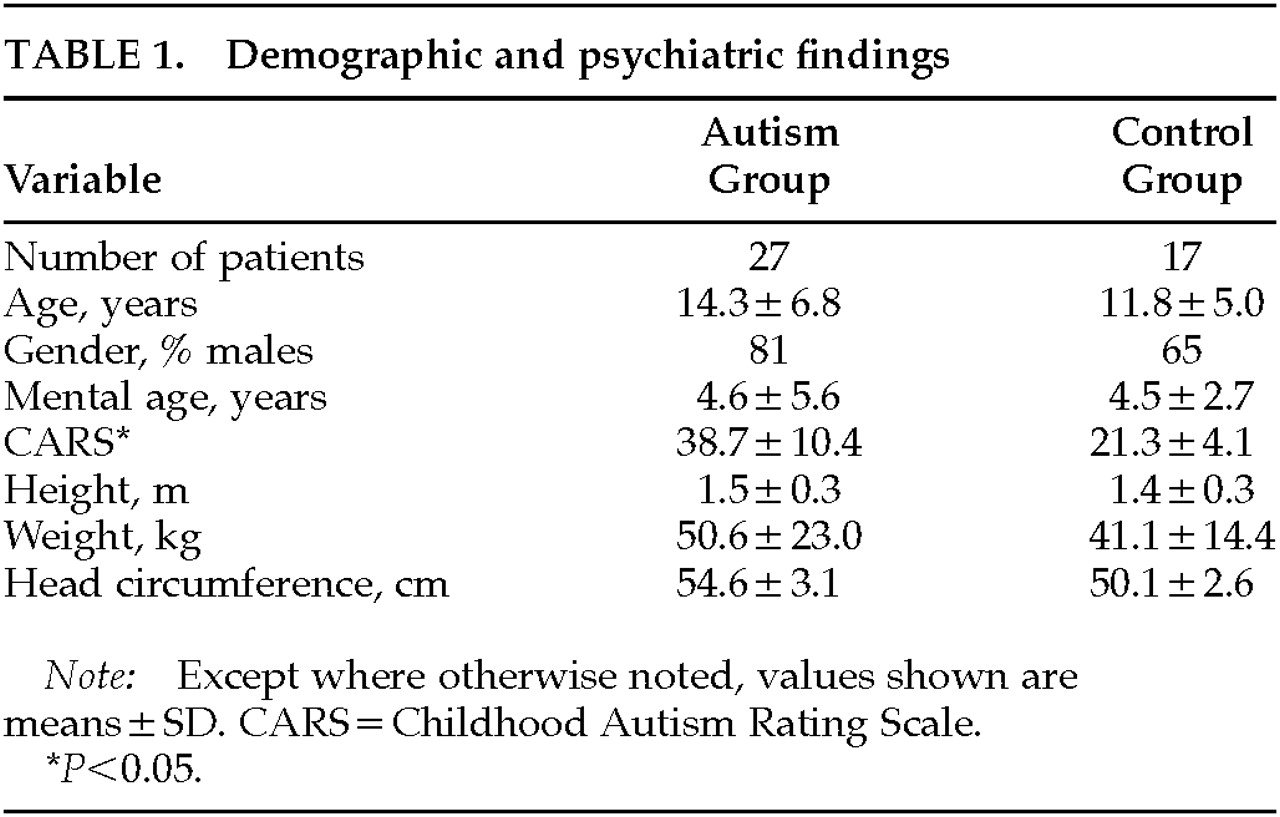
Demographic characteristics of the sample are shown in
Table 1. There were no significant differences in chronological age, nonverbal IQ, weight, height, or head circumference between autistic patients and control subjects. Eighty-one percent of autistic patients and 65% of control subjects were males.
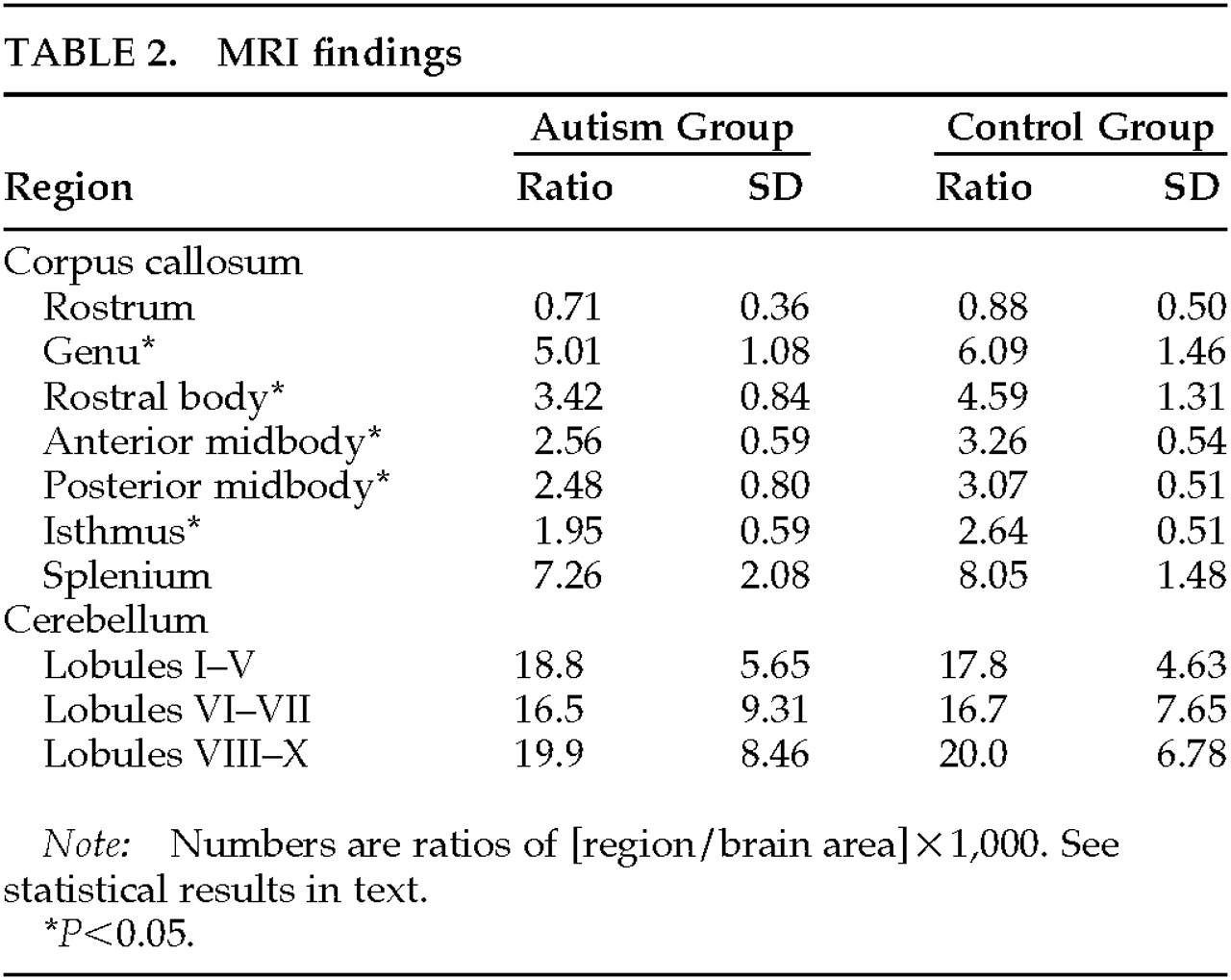
The results of analyses of CC and cerebellar size are shown in
Table 2. A MANOVA for the different regions of the CC showed a significant main effect (Wilks' lambda=0.54, df=7,36,
P<0.001): total CC area was significantly smaller in autistic subjects. On individual comparisons, autistic subjects showed a significantly smaller genu (
F=7.69, df=1,41,
P<0.01), rostral body (
F=12.9, df=1,41,
P<0.001), anterior midbody (
F=13.4, df=1,41,
P<0.0001), posterior midbody (
F=6.11, df=1,41,
P<0.05), and isthmus (
F=14.6, df=1,41,
P<0.001) than the control group. No significant between-group differences were found for the rostrum (
F=2.23, df=1,41,
P=0.14) or the splenium (
F=1.44, df=1,41,
P=0.23).
A MANOVA for cerebellar measures showed no significant main effect (Wilks' lambda=0.98, df=3,39, P=0.89), and no significant effects for cerebellar regions I–V (F=0.02, df=1,41, P=0.87), VI–VII (F=0.16, df=1,41, P=0.68), and VIII–X (F=0.00, df=1,41, P=0.92). Exclusion of the 4 patients with a history of seizures did not change the statistical findings.
DISCUSSION
Our study replicates and extends the findings by Egaas et al.
4 and Piven et al.
5 by demonstrating a significantly smaller size of the CC (most marked in the body region) in lower functioning autistic subjects, and it extends the findings of several other groups showing no evidence for a selective hypoplasia of cerebellar lobules VI–VII. In comparison with previous imaging studies in autism, two strengths of this study should be noted. First, whereas the study by Piven et al. was limited to the examination of three subregions of the CC (anterior, body, posterior), in this study we examined seven subdivisions of the CC. Regional subdivisions of the CC can be roughly associated with cortical regions
23 to potentially improve localization of abnormalities. Prior studies have demonstrated that cortical regions can be mapped to specific subregions of the corpus callosum,
24,25 and these seven different parts of the callosum have not been measured in autistic individuals. Second, this was a study of mentally retarded autistic individuals, a population for which few neuroimaging studies have been reported. A limitation of this study should also be mentioned: volumetric scans were not acquired, raising potential problems due to variation in head position between subjects.
A major question in this study is the significance of the callosal size abnormalities in autism. The first issue is whether CC abnormalities represent hypoplasia or atrophy. The question has no definite answer since to our knowledge there are no longitudinal MRI studies of callosal size in autistic individuals. The CC develops between gestational weeks 8 and 17, and insults during its formation may produce not only severe CC abnormalities (e.g., complete or partial CC agenesis), but other brain malformations as well (e.g., eversion of the cingulate gyrus
26). Since none of these gross abnormalities are evident in MRI studies of autistic individuals, early disorders of CC development are probably unlikely. Abnormal development of the CC may also result from abnormal migration of neuroblasts from the germinal matrix area of the lateral ventricles to their final cortical placement, which takes place between the first trimester and the end of the second trimester. This deviation may result in a fully developed CC with narrowing in those sections that consist of fibers projecting from cortical areas with migration abnormalities.
26 Hayakawa et al.
27 assessed the development of the CC with MRI and reported an exponential increment in CC area during the first 4 years of life and a slower growth until ages 10–12. Thus, the first 4 years of life may be an additional period of potential CC abnormal development among autistic individuals. Piven et al.
5,28 pointed out a potential discrepancy between their findings of reduced callosal size
5 and enlargement of the parietal, temporal, and occipital lobes.
28 They suggested several hypotheses to reconcile these findings, such as a relatively larger increment of ipsilateral versus contralateral connections, a greater volume of non-neuronal cortical tissue, or an increased number of cortical neurons that do not project axons to the callosum.
Size abnormalities in cerebellar vermal lobules of autistic individuals have been examined primarily among high-functioning subjects. Courchesne et al.
15 reported neocerebellar hypoplasia in most autistic individuals (87%) and hyperplasia in a smaller proportion (13%). However, this finding has not been replicated in studies by other authors,
17 who have pointed out methodological limitations in the Courchesne et al. study. Similarly, our group of low-functioning autistic individuals did not differ significantly in cerebellar measurements from mental age–comparable control subjects, supporting other authors' findings
11,12,29,30 and suggesting that the neocerebellar hypoplasia reported by Courchesne et al.
6 may have been the result of problems in study design (e.g., selection of a supernormal control group from a convenience sample of MRIs that were previously read as normal) or analysis (e.g., failure to adjust for potential confounders such as IQ).
In summary, we found significant size reductions in specific regions of the CC of autistic individuals with mental retardation as compared to a nonautistic control group with similar mental age. Whether these anomalies are an epiphenomenon of more widespread brain damage or have a more specific etiologic and pathologic role in autism is a question that warrants examination in future studies.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Raúl Carrea Institute of Neurological Research, and the Fundación Pérez Companc. The authors thank Alejandra Del Val, M.D., and Indhira Del Giudice, Ph.D., for providing psychiatric and neuropsychological assessments.