Cognitive disturbance has long been recognized as a fundamental feature of schizophrenia. Impairments of attention, verbal fluency, memory, and executive functions are widely reported and appear to be persistent.
1 The probable influence of these cognitive impairments on course, positive and negative symptoms, and social and vocational outcome is widely accepted yet far from understood.
2,3 Evidence from treatment response studies suggests that typical neuroleptics have minimal value in improving these impairments and may actually worsen some cognitive functions.
4 Recently, the success of clozapine in the treatment of refractory symptoms has created interest in this drug's impact on cognitive features and how this impact might translate into functional outcomes. Unfortunately, understanding of the cognitive effects of clozapine is at an early stage.
5 To date, only a few investigations have examined the effect of clozapine on cognitive functioning.
6–13 A summary of these is provided in
Table 1.
Although small samples and varying designs, assessments, and follow-up periods have made generalizations difficult, some evidence of meaningful drug-related cognitive change is emerging. For example, verbal fluency and verbal memory tend to improve during clozapine treatment. On the other hand, visual memory and set shifting have been reported to worsen.
Duration of clozapine treatment before assessment, dosage, and other treatment-related factors may contribute to these changes. However, an exclusive research focus on these possible factors may have obscured the connection of cognitive changes to functional responses, such as the influence changes may have on discharge or employment.
Given the heterogeneity of schizophrenia, it seems reasonable to expect that in any group of schizophrenic patients selected for clozapine treatment there will be a variety of responses. Indeed, individual variability seems marked in both the clinical and the cognitive responses to clozapine. Heterogeneity of response will tend to be masked by data analyses that focus on the overall changes in functioning of a group of treated patients. If the effect of clozapine is to produce improvement in some patients but no change, or exacerbation of impairment, in others, the net effect in the group as a whole is likely to be no significant change between pretreatment and posttreatment means.
The complexities and risks of clozapine treatment are strong reasons to try to identify the characteristics of responders and nonresponders to this medication, and most especially to identify the characteristics that distinguish the two groups at baseline assessment before the treatment begins.
With these considerations in mind, the present study was conducted in an attempt to address certain issues. Our first aim was to identify the pretreatment characteristics (clinical, cognitive, and motor/sensory) that distinguish between patients treated with clozapine who improve to the point of discharge from hospital and those who do not. Information on this point will necessarily be post hoc because there is no extant theoretical model from which specific changes might be predicted in advance. This, in turn, means that whatever differences are found will require validation through replication by research on other samples.
The second aim was to identify changes in the variables of interest that occur during the course of treatment, differentiating between those that are related to discharge or continued hospitalization and those that are not. The same cautions regarding post hoc analyses apply to this issue.
METHODS
Subjects
This report is based on 54 schizophrenic (
n=41) and schizoaffective (
n=13) patients who completed a trial of clozapine at a state hospital. All treatment-resistant schizophrenic or schizoaffective patients between the ages of 18 and 65 years in this facility with a history of poor response to at least two trials of standard neuroleptic medication were referred for and received a clozapine trial. The ward psychiatrist made diagnoses using DSM-III-R criteria,
14 and these diagnoses were confirmed by the authors using the Schedule for Assessment of Affective Disorders and Schizophrenia (SADS),
15 the Scale for the Assessment of Negative Symptoms (SANS),
16 the Scale for the Assessment of Positive Symptoms (SAPS),
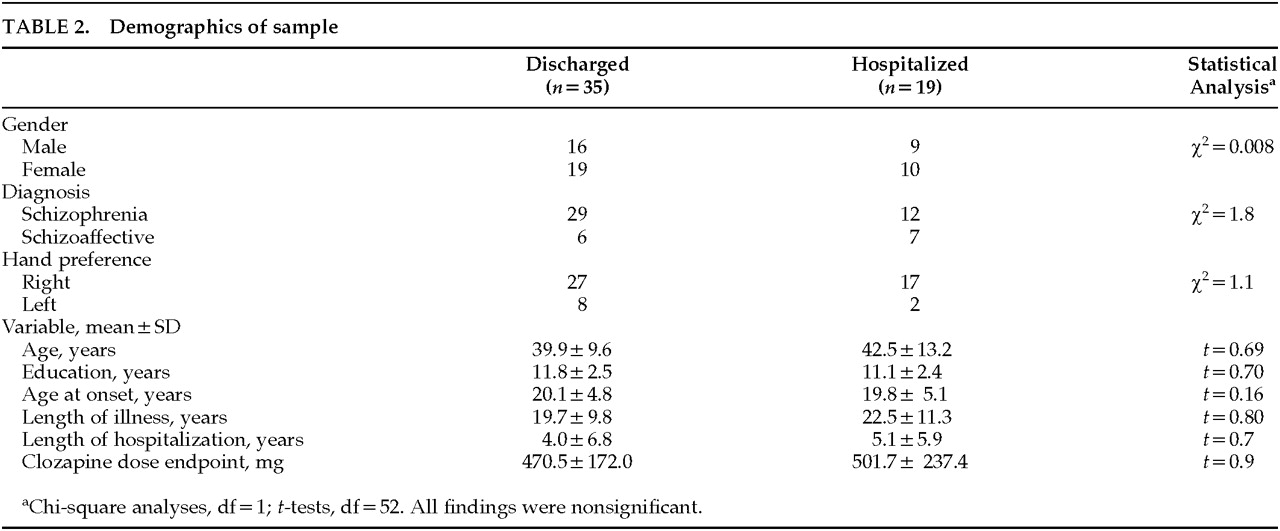
17 and a review of the clinical record. All patients and/or their guardians gave informed consent. The schizophrenic and schizoaffective subjects did not differ on any baseline variables, so all patients were treated as one sample. Demographics of the subjects are shown in
Table 2.
Design
This study was a 1-year, open-label, flexible-dose trial with patients serving as their own controls. Treatment with clozapine began at 25 mg or 12.5 mg under the direction of the attending psychiatrist. Dosages were increased once or twice per week by increments of 25 mg to 50 mg to a target dose in the range of 300 to 600 mg per day. When clinically indicated, further adjustments in the dose were permitted. Where possible, all additional medications were reduced or discontinued in the titration phase of the trial; however, in some cases patients continued to receive concomitant medication.
Prior to the beginning of clozapine treatment, staff collected baseline measures during the optimal treatment regimen. These measures were repeated at least every 6 months thereafter and immediately prior to a subject's discharge, if discharge occurred.
Measures
Clinical Assessment Rating scales were used to assess psychiatric symptoms and motor side effects. Psychopathology was measured with the Brief Psychiatric Rating Scale (BPRS).
18 Positive and negative symptoms were rated by using summed scores from the SAPS
17 and the SANS,
16 respectively. A thought-disorder rating was derived from a combination of individual items from the SANS (poverty of content of speech) and SAPS (derailment, incoherence, and illogicality). Extrapyramidal features were measured with the Simpson-Angus Rating Scale
19 and other dyskinesias with the Abnormal Involuntary Movement Scale (AIMS).
20The reliability among three raters for the total score on the BPRS assessments was 0.86, as determined by intraclass correlation coefficients. The interrater reliability was also adequate (no coefficient less than 0.80) for the SANS, SAPS, AIMS, and Simpson-Angus rating scales.
Global cognitive functioning was assessed with the Mini-Mental State Examination (MMSE).
21 Additionally, an estimate of intelligence derived from the Wechsler Adult Intelligence Scale–Revised (WAIS-R
22) Vocabulary and Block Design subtests was obtained for subjects at baseline and at 6-month intervals.
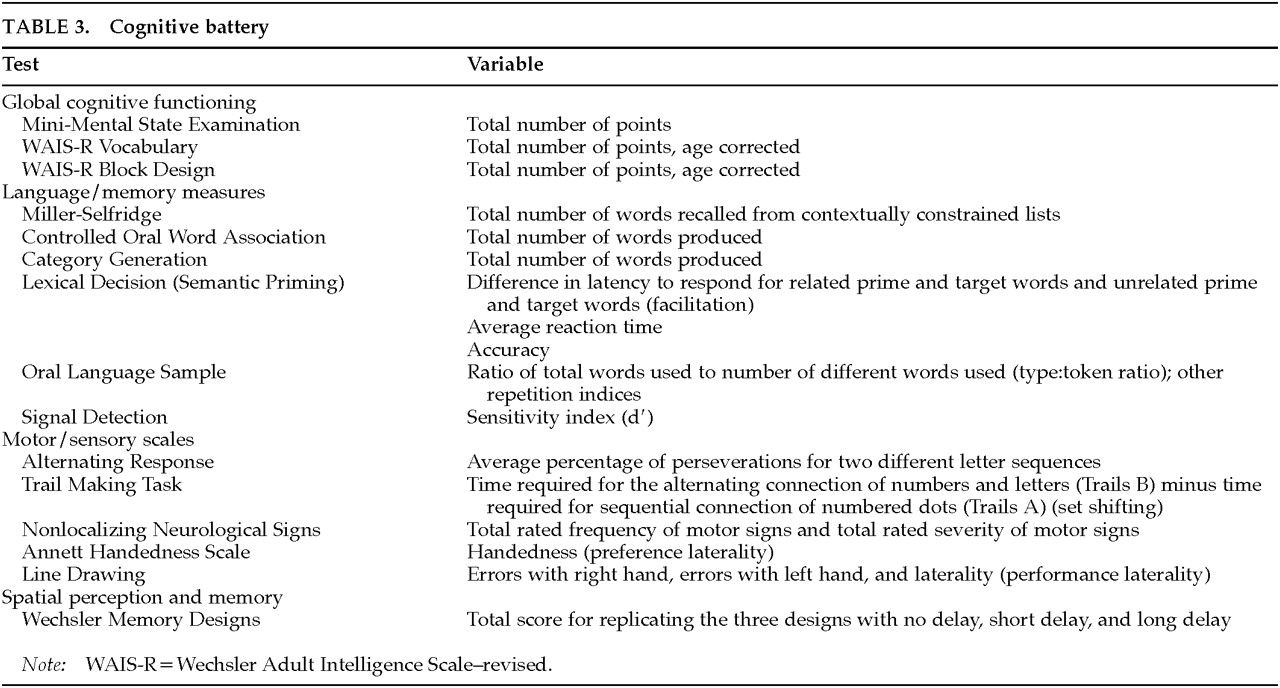
The rationale and a detailed description of the additional cognitive measures (listed in
Table 3) has been published elsewhere.
23 The tests included measures of language processing (Miller-Selfridge Recall Task;
24 Controlled Oral Word Association;
25 semantic priming;
26 oral language sample;
27 signal detection
28), motor and sensory functions (Alternating Response Task;
29 Trail Making Task;
30 Neurological Nonlocalizing Signs;
31 Line Drawing task;
32 Annett Handedness Scale
33), and spatial perception and memory (Wechsler Memory Designs
34).
With modification to accommodate the use of the 24-item BPRS,
35 symptomatic outcome was measured by using the BPRS improvement criteria used in the original multicenter trial.
36 According to that report, clinical improvement (i.e., the patient being a “responder” to clozapine) was defined by a 20% decrease in BPRS total score from baseline. This criterion was chosen to provide comparability of our finding with those used by Kane et al.
36 Functional outcome was defined by discharge status. Discharge decisions were made by the attending psychiatrists and clinical teams independent of the research staff. None of the patients were considered ready for discharge planning efforts prior to beginning clozapine therapy.
Baseline was defined as the assessment point immediately preceding the initiation of treatment. Endpoint was defined as the assessment immediately prior to discharge or, in the case of the hospitalized patient group, the 12-month assessment. The average endpoint did not differ between the two groups (mean±SD: discharged patients, 9.3±7.3 months; hospitalized patients, 12.0±0 months; t=1.4, df=52, P=0.15).
Analysis
The first analysis consisted of one-way analyses of variance (ANOVAs) for each variable, the independent variable being group status (hospitalized vs. discharged). Mean scores of the two groups were compared for the baseline assessments. This first comparison permits the identification of pre-treatment differences. In addition, to identify treatment related changes, one-way ANOVAs were conducted for each variable within each group, with the independent variable being point of assessment.
For the final analysis, for each subject, a change score (endpoint minus baseline) was produced for each variable. We were interested in how both baseline performance and change in performance following treatment related to discharge. Survival analysis techniques were used to predict likelihood of discharge based on both baseline and change scores for each clinical and cognitive variable.
RESULTS
Minimum significance level was established as P<0.05, with values greater than 0.05 but less than 0.10 reported as trends. Although there is no available model from which to predict specific baseline differences or changes during treatment, we might perhaps assume that any cognitive changes produced by treatment with a medication known to produce clinical improvement would also be in the direction of improvement. In the interests of caution appropriate to a post hoc strategy, we have not employed one-tailed significance levels.
In
Table 4 we present the means at baseline and change from baseline to endpoint for the two groups. We turn first to the baseline (pre-treatment) differences.
Pre-treatment Differences
As we have already seen in
Table 2, there were no significant differences between the two groups on any of the demographic variables. Nor were there any significant differences between the group means on any of the clinical rating measures. The group means of the following five measures from the cognitive assessment differed significantly: WAIS Full-Scale IQ, the Sensitivity measure from signal detection, two measures from the line-drawing performance test, and the Wechsler Memory Designs (total). In each case the pre-treatment score of the discharged group mean was in the direction of superior performance. It is possible to assign subjects into right-handed and left-handed groups based on performance on the line drawing test. Doing so revealed that significantly more patients from the discharged group (77%) than the hospitalized group (36%) showed a right-hand advantage (χ
2=6.9,
P=0.01).
Changes During the Treatment Period
At the outset we should note that none of the five score differences seen at baseline between the groups changed significantly for either group over the course of treatment.
During the treatment period, four clinical measures showed significant improvement in the discharged group. These were the BPRS score, SAPS total, SANS total, and Simpson-Angus total. Two other measures, Thought Disorder (SANS/SAPS) and AIMS total, showed trends toward improvement (P<0.10). For the hospitalized group, only SAPS and SANS significantly improved; three of the other four measures showed trends toward improvement.
The general summary of these analyses is that clozapine produced improvement in clinical variables. The differences in significance levels between the two groups may reflect the influence of the smaller sample size of the hospitalized group, with the implication that this difference might disappear with larger samples. Alternatively, it may reflect the possibility that the clinician's decision to discharge a patient is substantially influenced by clinical observation of the kinds of changes that the ratings measure. With this in mind, we compared the percentage reduction in mean BPRS score in the two groups. The discharged group's mean percentage reduction was 16±19, while that of the hospitalized group was 7±32. This difference does not achieve significance (P=0.32).
An examination of the individual patients' percentage improvement in BPRS score, using the clozapine multicenter criterion
36 of a 20% reduction in score, showed that the 23 patients who met this criterion were no more likely to be discharged than the 31 who failed to reach it (
n=54; χ
2=0.46, df=1,
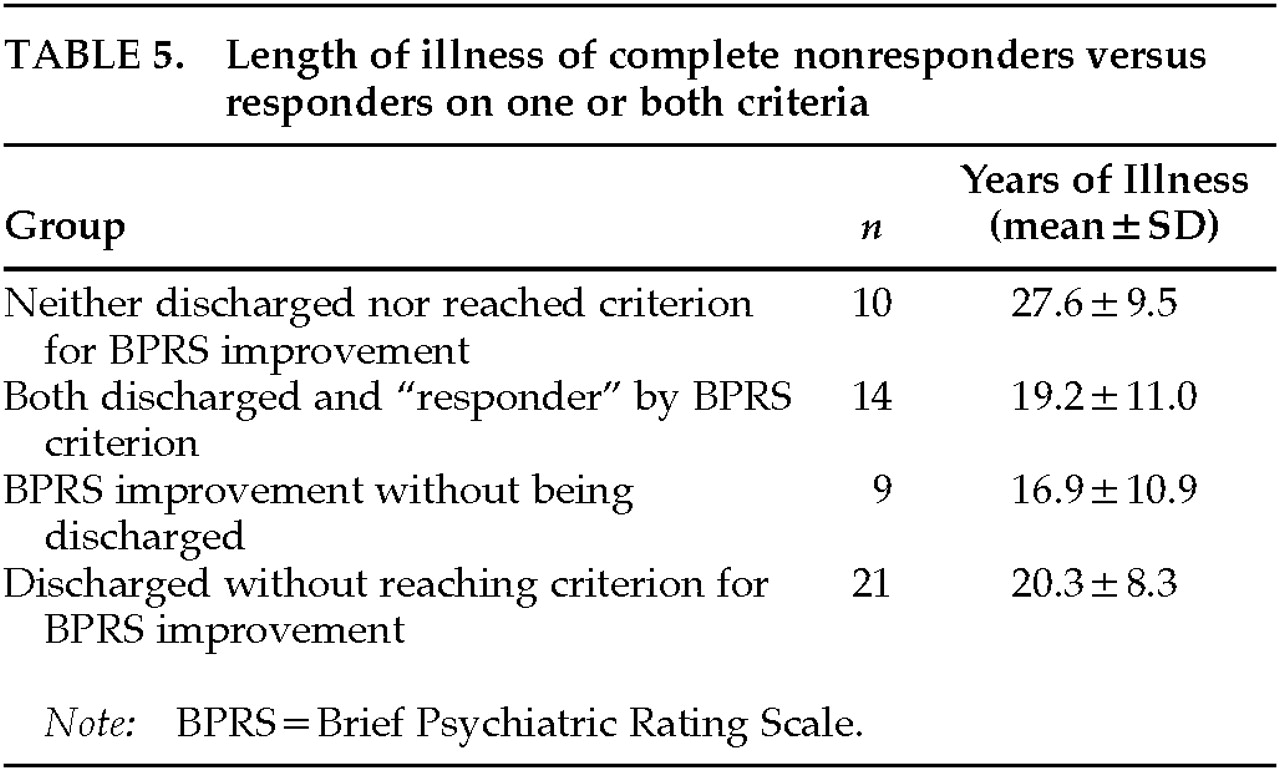
P>=0.6). We examined the demographic characteristics and other measured variables of the concordant and the nonconcordant cases (i.e., patients in whose case the decision whether or not to discharge corresponded to the multicenter criterion for improvement, and patients where the discharge decisions were not consistent with that criterion). A two-way ANOVA (discharge×multicenter criterion) was employed. The one finding was a significant interaction with the variable length of illness (LOI) (
F=6.20, df=1,50,
P=0.016;
Table 5). The product-moment correlation between LOI and percentage change in BPRS scores for the group of 54 patients was –0.32 (
P=0.02).
The ANOVA interaction was such that 10 hospitalized patients who had also failed to meet the multicenter criterion for improvement had a greater mean length of illness (27.6 years) than the 44 who a) had met the criterion and been discharged, b) had met the criterion and not been discharged, or c) did not meet the criterion but had been discharged. Mean LOI did not differ within these latter subgroups, and it is clear that the significant correlation is due to the influence of this small group of chronic patients. This long-term chronic group did not differ significantly from the other subgroups on any measured clinical or cognitive variable. As a matter of clinical interest, only 2 of the 10 unimproved patients had LOIs of less than 20 years, compared with 23 of the 44 remaining patients.
Bearing in mind the limitations of sample size here, these data suggest that clozapine treatment of patients with very long LOIs may be unlikely to result in improvement by either of the measures (discharge or the multicenter criteria) that were employed in the present study.
The hospitalized group showed no significant changes in any of the cognitive assessment variables over the course of treatment. There was a trend for category generation to decline, from 12.7 to 9.6. In the discharged group, Controlled Oral Word Association and set shifting in the Trails test improved significantly, and reaction time in the lexical decision task showed a trend to improvement.
Some patients were receiving either one medication or a combination of medications, including lithium, antidepressants, benzodiazepines, and anticonvulsants, at baseline and/or endpoint. Chi-square analysis revealed minimal differences among the distribution for these medications between the two groups; furthermore, rerunning the analyses excluding subsets of patients on selected medicines revealed no influence of these additional medications on the main measures.
Predicting Discharge
We used survival analysis to predict discharge status from baseline functioning and medication response. A proportional-hazards model was fitted to the data, using month of discharge as the event time. Along with demographic information, baseline and change scores for each clinical and cognitive variable were entered singly into the regression model. Two cognitive variables had significant predictive value for discharge: baseline Wechsler scores (β=0.056, P=0.003) and change in number of category words generated from baseline to endpoint (β=0.088, P=0.04). The positive coefficient of these variables indicates that increasing scores are associated with increased risk of event occurrence—in this case, discharge. Demographic and clinical variables failed to show any predictive utility.
DISCUSSION
We examined the clinical and cognitive effects of clozapine among severely ill, hospitalized schizophrenic and schizoaffective patients. Consistent with earlier reports,
19 clozapine treatment had positive effects on negative and positive symptoms and general psychopathology. In addition, clozapine may reduce parkinsonian features and abnormal involuntary movements.
Generally consistent with earlier reports (see
Table 1), there were significant cognitive improvements among those who were discharged. These included improved verbal fluency, reaction time, and set shifting. The question then arises, what is the significance, both clinically and practically, of this cognitive improvement?
A clue to the answer may lie in the observation that clozapine treatment also resulted in a meaningful and unexpected functional improvement, namely discharge from the hospital. Prior to the start of this study, most patients in the sample had been hospitalized continuously for years, and prospects for discharge were dim despite a systematic program of hospital-based rehabilitation. During this trial, 35 of 54 patients (65%) improved enough to be discharged.
As noted, clinical symptoms likely had some influence on the clinician's decision to discharge a patient; appearance and behavior clearly will influence certain clinical decisions. Our data support the possibility that cognitive features may have as much, if not more, influence on some outcomes. Our analysis revealed that the cognitive performance of the discharged and hospitalized groups differed at baseline: discharged patients were better than their hospitalized counterparts at baseline on the WAIS-R Full Scale IQ and the Wechsler memory task; were more sensitive in remembering and identifying presented words (signal detection); and as a group showed more of a right-hand performance advantage on the line-drawing task.
In fact, cognitive performance may have more impact on discharge readiness than does clinical symptom improvement. We attempted to examine this issue by predicting discharge from clinical symptomatology and cognitive performance. We were unable to find a predictive connection between the multicenter criteria
36 and being discharged; clinical “responders” were not discharged more frequently than nonresponders. The results of our survival analysis bolster the claim that cognitive functioning is more important for discharge in this sample than is clinical symptom response. Although demographic and clinical features failed to predict discharge, two variables did: visuospatial memory at baseline, as assessed by the Wechsler Memory Scale designs subtest, and improvement in verbal fluency (category prompt) from baseline to endpoint. This finding is also consistent with observations of others who have reported that cognitive functioning is a better predictor of outcome than symptomatology in schizophrenia.
2,5 The implication of such findings is that the pathophysiologies of cognitive deficits and symptomatology in schizophrenia may be independent of each other.
It might be argued that some patients did not show cognitive improvement because of certain side effects (e.g., motor difficulties or sedation). The available data do not support this contention, since there were no relationships noted between AIMS and Simpson-Angus measures and cognitive measures, nor were there differences between the patient groups on these measures. The procedural effects of excluding data from patients unable to complete a task, and the fact that there were no differences between the discharged and hospitalized patients on the MMSE, which includes a measure of general alertness, suggest that sedation was not a factor influencing these results.
To sum up, clozapine appears to improve symptoms and cognitive performance. Moreover, cognitive performance appears to be more important than clinical symptoms as a predictor of functional outcome. This should not be entirely surprising, considering that researchers have begun to recognize the important connection between cognitive abilities and functional outcome in psychiatric patients. For example, memory—particularly verbal memory—has been suggested by other researchers
3 as an important “threshold” or rate-limiting factor in achieving certain community, social, and skill-related outcomes. Such a conceptualization of cognitive abilities is consistent with our findings.
One plausible way to consider these findings is to see them as a reflection of diffuse disturbance in brain functioning brought on by schizophrenic illness. Better lateralization, relatively higher IQ, and relatively better memory at baseline all suggest that discharged patients had less severe brain abnormality prior to treatment. Improvement in cognition that leads to discharge may reflect an impact on remediable brain functioning. The reason some of the patients may not have improved as much as others may have to do with the extent of the disturbance.
CONCLUSION
Although clozapine treatment effectively reduced symptoms in this sample of chronic, treatment-resistant subjects, a finding consistent with prior reports, the present study demonstrated that clozapine also has important effects on cognition. Further—and apparently independently of clozapine's effect on symptoms—these cognitive changes have an important role in influencing at least one functional outcome, that of discharge from long-term hospitalization. Interestingly, baseline cognitive performance on visuospatial memory and improvement in category generation, a form of verbal fluency, predicted discharge, whereas clinical symptoms at baseline and in response to clozapine were not predictors of discharge. The contribution of cognitive response to treatment outcome is gradually being defined, and it must be recognized for its practical value and its implications for understanding schizophrenia.
ACKNOWLEDGMENTS
This work was presented at the annual meeting of the New Clinical Drug Evaluation Unit, Boca Raton, FL, June 1998.