Although deficits in anterograde memory are the hallmark of Alzheimer's disease (AD), the prevalence and clinical correlates of remote memory deficits in this disease have been less extensively studied. Wilson and Cockburn
1 assessed AD patients with a facial identification task and a test of recall and recognition of public events and famous people of past decades. They and others
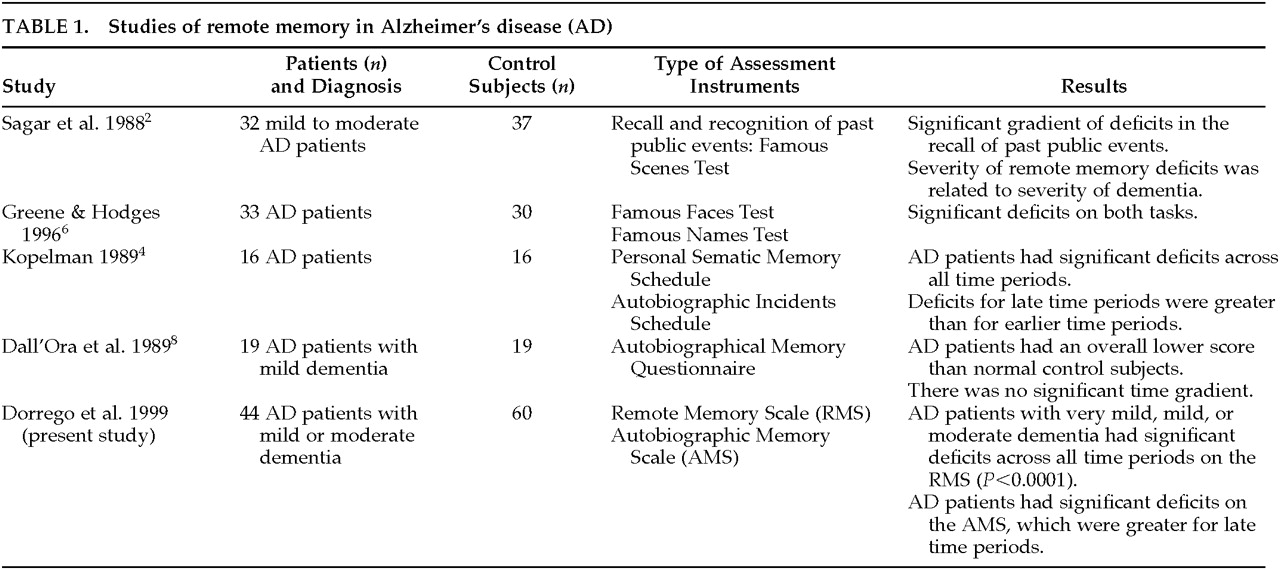
2–5 found that AD patients performed significantly worse than normal control subjects across all decades in both tests. Sagar et al.
2 examined remote memory in patients with mild to moderate AD, using recall and recognition tests of public events. They found a gradient of deficits in the recall of past public events (remote events being less affected than recent events), and the severity of the memory deficit was related to the severity of dementia. In a recent study, Greene and Hodges
6 assessed AD patients with a neuropsychological battery designed to assess face recognition, identification, and naming; they found that even those with mild dementia had significant deficits on all tests of remote memory. A recent study by Storandt et al.
7 assessed dementia and control patients with a recognition task for remote events. Patients with dementia performed worse than control subjects, but there was no temporal gradient.
Autobiographical memory (ABM) in AD has been typically assessed by asking patients to retrieve and date autobiographical memories in response to a list of cue words.
3–4 One limitation of this technique is that patients might report memories only for restricted time periods and not from the whole life span. Moreover, memories produced by patients are not verified for accuracy with other sources familiar with the patient's life, and whether the memories produced are real or fabrications cannot easily be determined in those studies. The assessment of ABM was improved by Kopelman,
4 who examined ABM in a group of 16 patients with AD by using two instruments: the Personal Semantic Memory Schedule, which probes patients' knowledge of facts about their past, and the Autobiographic Incidents Schedule, which probes patients' capacity for producing a memory of a specific incident. Kopelman
4 found that AD patients had significant deficits across all time periods as compared with age-comparable normal control subjects, although deficits for late time periods were greater than for earlier time periods. Dall'Ora et al.
8 examined 19 AD patients with mild dementia with an ABM task that included five questions for each of the following three periods: childhood and adolescence, early adulthood, and late adulthood. AD patients had an overall lower score than a group of age- and education-matched normal control subjects, but there was no significant time gradient; that is, recall for early and late time periods was similar. Dall'Ora et al.
8 found no significant correlations between scores on the ABM and performance on tests assessing verbal span, episodic and semantic memory, or abstract reasoning and suggested that ABM deficits may result from defective organization of search. This hypothesis was further investigated by Greene and colleagues
9,10 and Kopelman;
11 both found a significant association between tests of executive functions and ABM, as well as a modest correlation between tests of retrograde and anterograde memory. However, correlations with other neuropsychological tasks were not calculated, and whether other cognitive domains are also relevant to deficits in ABM was not determined. The finding of Greene et al.
9 that ABM deficits were also present after cued questions led the author to suggest that ABM deficits may result from both degradation of individual memory traces and retrieval deficits. A better approach to the issue of retrieval-versus-degradation of memory traces is to examine performance on free recall and recognition versions of the same questions, but to our knowledge this pairing has not been assessed. Studies of remote memory in AD are summarized in
Table 1.
The present study examined remote memory in a series of AD patients with very mild, mild, or moderate dementia, using a newly designed set of tests that included the Remote Memory Scale (RMS) for famous people and well-known events and the Autobiographical Memory Scale (AMS). We also assessed our patients with a comprehensive neuropsychological battery, which allowed us to examine correlations between performance on specific cognitive domains and deficits of remote memory.
METHODS
Patients and Procedure
A consecutive series of 44 patients meeting the NINCDS-ADRDA
12 criteria for probable AD participated in the study. Twenty-one patients completed both the RMS and the AMS, 19 patients completed only the RMS, and 4 patients completed only the AMS. Patients with a history of stroke, one or more focal lesions on MRI (T
1-weighted), or a Hachinski Ischemic score greater than 4 were excluded from the study.
After informed consent was obtained, patients and control subjects were assessed with the Remote Memory and Autobiographical Memory Scales. Because of methodological limitations, fewer patients could be assessed with the AMS. AD patients were also given a comprehensive neuropsychological evaluation as described below.
Remote Memory Scale
The pilot RMS consisted of 80 famous names and 80 well-known events belonging to the 1950s, '60s, '70s, and '80s (20 names and 20 events from each decade) and consisted of free-recall and recognition sections. Thus, the free-recall section consisted of 160 questions presented in a randomized order. The recognition section included all of the 160 randomized questions and four options for each question (the correct answer, two related distractors, and one irrelevant answer).
The pilot RMS was assessed in 20 normal control subjects (mean age±SD= 49.5±18.3 years, range 24–83; 55% females; 50% high [≥10 years] and 50% low [<10 years] educational level). The questions that showed a significant difference for gender (4) or educational level (2), as demonstrated by t-tests using gender or educational level as grouping variables, were deleted, and the final RMS version included only those questions with a success rate (percentage of correct answers) between 50% and 95%. (This wide cutoff was selected to avoid ceiling effects for the control group and floor effects for the AD group.) From those questions meeting this criterion, we randomly selected 40 questions to obtain two sections (free recall and recognition), each employing the same 40 questions (10 for each decade: 5 famous names and 5 well-known events).
Figure 1 shows the performance of young normal control subjects (
n=10; mean age=25.4±1.5 years, range 24–35) and older normal control subjects (
n=10; mean age=58.2±1.3 years, range 36–83) on the free recall section of the pilot RMS. A three-way analysis of variance (ANOVA) with repeated measures (
factor 1: group [young vs. old];
repeated measures: decades [1950s to 1980s] and item [famous names vs. well-known events]) demonstrated a significant group effect (
F=13.2, df=1,18,
P=0.001): older control subjects remembered significantly more items than young control subjects; a significant effect for decades (
F=6.63, df=3,54,
P<0.001): there was a significantly lower recall for the 1950s compared with the other three decades; and the expected significant group×decade interaction (
F=10.0, df=3,54,
P<0.0001): young control subjects had a significantly lower recall for the 1950s, '60s, and '70s, but not for the '80s, than older control subjects. There were no significant effects for test (i.e., free recall vs. recognition), test×decade interaction, or group×test×decade interaction. The lack of a significant test effect on the free recall section may be explained by a ceiling effect for the old control subjects on the free recall section and a floor effect for the young control subjects to early-decades stimuli. Test-retest reliability was assessed by giving the RMS to 10 AD patients (MMSE mean score=24.2±5.4) twice, 1 month apart, and proved to be high (
r=0.98). No reliability measures were calculated for the control group.
Autobiographical Memory Scale (AMS)
The pilot AMS consisted of 100 questions about specific dates, places, events, individuals, etc., from personal history from five periods: childhood (6 to 12 years), adolescence (12 to 18 years), early adulthood (18 to 35 years), adulthood (35 to 60 years), and recent events. There were 20 questions for each period, and each question included a confidence scale that was rated as “completely sure,” “almost sure,” “not very sure,” or “unsure.”
The pilot AMS was assessed in first-degree relatives of 10 normal control subjects (not assessed with the RMS; usually a sibling; one informant per control individual; age range 52 to 74 years; 60% females). For the final AMS version, we selected only those questions for which the agreement between the normal control subjects and their respective relatives was above 90% and the confidence scale score was either “almost sure” or “completely sure.” Based on these criteria, the final version of the AMS consisted of 39 questions: 6 assessing childhood, 6 adolescence, 9 youth, 9 adulthood, and 9 recent events. There was a free recall section, where patients answered each question, and a recognition section, which used the above 39 questions with three different answers: the correct one (provided by the family member) and two semantic distractors. The percentage of questions correctly answered for each period serves as the metric. The test-retest reliability of the scale, examined by giving the scale twice with a 1-month interval to 10 AD patients (MMSE mean±SD=22.5±4.6), was high (r=0.96). This was not the same group of AD patients used to test reliability for the RMS. We further examined the reliability of the scale by calculating its internal consistency, which was also high (Cronbach's α=0.81).
Neuropsychological Battery
AD patients were also assessed with the following neuropsychological battery by examiners blind to results on the RMS and AMS scales:
•
Buschke Selective Reminding Test:13 This test measures verbal learning and memory on a 10-trial list-learning task.
•
Digit Span:14 This test examines auditory attention.
•
Wisconsin Card Sorting Test:15 This test measures the ability to acquire new concepts and shift sets.
•
Controlled Oral Word Association Test:16 This test examines access to semantic information with time constraint.
•
Boston Naming Test:17 This test measures the ability to name line drawings of high and low frequency objects.
•
Token Test:18 This test measures comprehension and execution of commands of increasing complexity.
•
Raven's Progressive Matrices:19 This test measures reasoning in the visual modality.
•
Mini-Mental State Examination (MMSE):20 The MMSE is an 11-item examination found to be valid and reliable in assessing a limited range of cognitive functions in a global way.
Statistical Analysis
Statistical analysis was carried out by using means and SDs, analysis of variance (ANOVA) with repeated measures, and post hoc t-tests. Regressions were calculated by using a forward stepwise regression analysis. Frequency distributions were calculated with chi-square tests and a Yates' correction for expected cell sizes less than 5. All P-values are two-tailed.
RESULTS
Study 1: Memory for Famous People and Well-Known Events
Demographic Findings:
We assessed with the RMS a group of 40 AD patients and a group 40 normal control subjects (not the same control subjects assessed for the pilot RMS study). Control subjects were spouses of AD patients or healthy elderly volunteers from the community.
No significant differences were found in age, gender, and years of education between AD patients and control subjects (mean years of age: AD, 72.0±7.3, control, 69.6±11.5; gender (% females): AD, 48%, control, 65%; mean years of education: AD, 10.7±4.5, control, 11.8±5.4).
Remote Memory Scale:
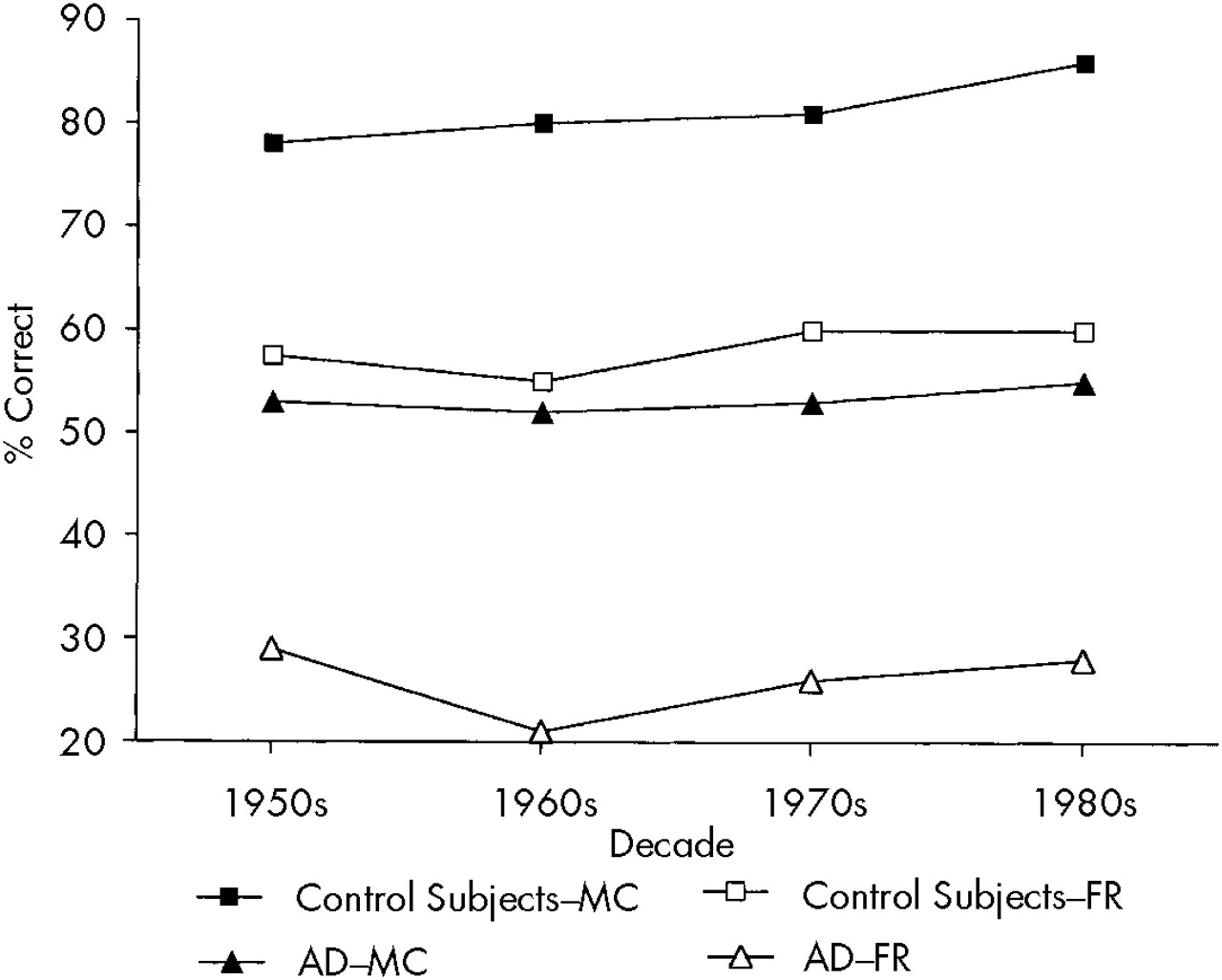
Figure 2 shows findings on the free recall and recognition sections of the RMS for AD and control subjects. A three-way ANOVA with repeated measures (
factor 1: group [AD vs. control subjects];
repeated measures: decades [1950s, '60s, '70s, and '80s], and test [free recall vs. recognition]) showed a significant group effect (
F=38.1, df=1,78,
P<0.0001): AD patients had a significantly lower score on the RMS than normal control subjects; a significant test effect (
F=281.9, df=1,78,
P<0.0001): the free recall section was significantly more difficult than the recognition section for all subjects; and a significant effect for decades (
F=5.28, df=3,23,
P<0.05): there was a significantly lower recall for the 1950s and '60s than for the '80s (
P<0.01 and
P<0.0001, respectively) for the overall group of AD patients and control subjects. On the other hand, there were no significant group×decade, group×test, or group×decade×test interactions.
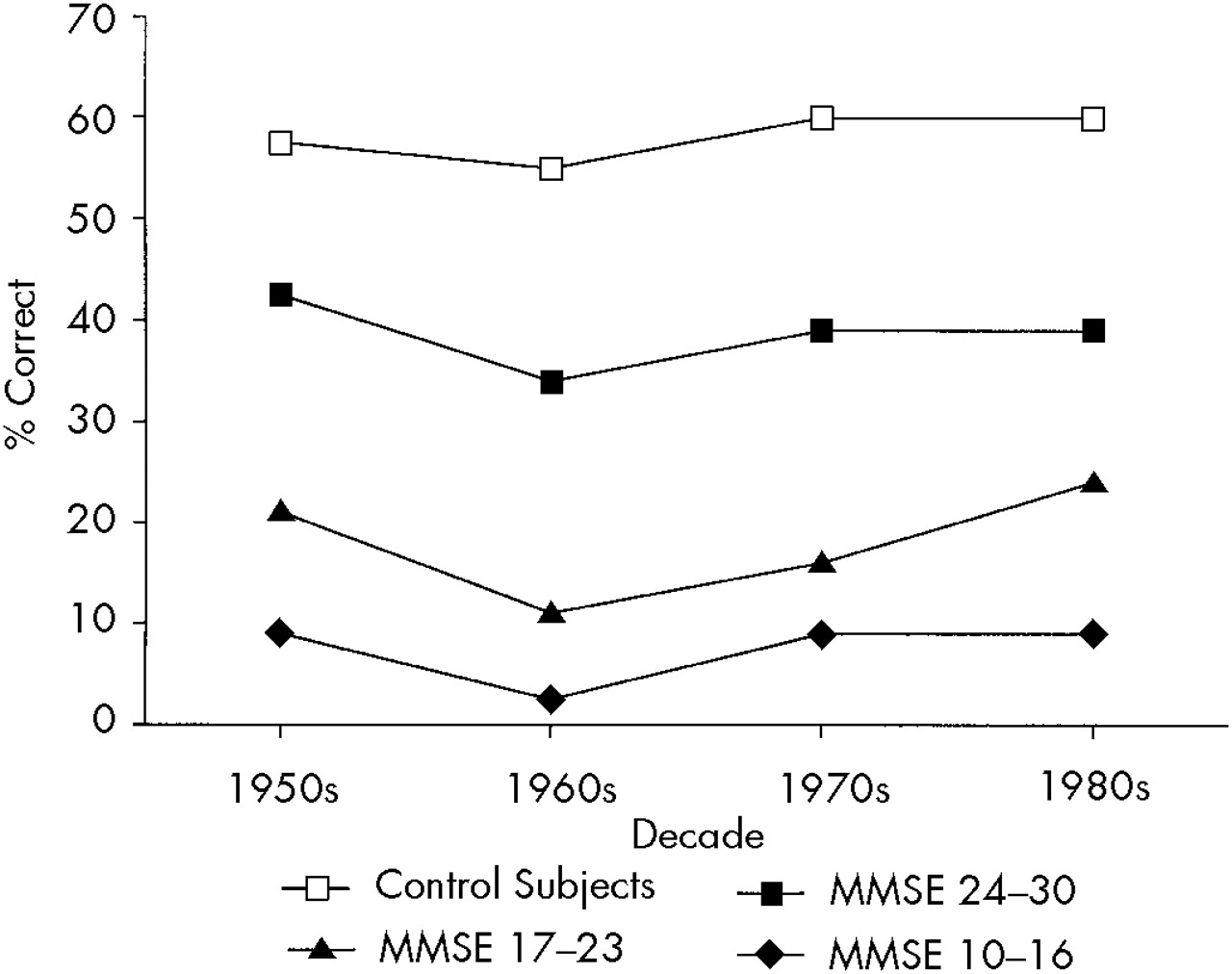
To examine whether the profile and severity of remote memory deficits were related to the severity of dementia, we separated AD patients into groups with very mild (MMSE scores 24–30;
n=20), mild (MMSE scores 17–23;
n=10), or moderate dementia (MMSE scores 10–16;
n=10). A three-way ANOVA with repeated measures (group×decade×test) showed a significant group effect (
F=24.4, df=3,76,
P<0.0001): normal control subjects had a significantly better performance than the three AD groups, which were also significantly different from each other (
Figure 3). There also was the expected significant effect for test (
F=232.6, df=1,76,
P<0.0001): recognition better than free recall, and a significant effect for decades(
F=3.84, df=3,228,
P<0.01): there was a significantly worse recall for the 1950s and '60s than for the '80s for the overall group of AD patients and control subjects.
Neuropsychological Correlates of Remote Memory in AD:
A forward stepwise regression analysis was calculated, using RMS scores as the dependent variable and age and neuropsychological scores as the independent variables. Results of the overall regression analysis were statistically significant (R2=0.60, F=37.0, df=3,36, P<0.0001), and both the Boston Naming test (R2=0.48, P<0.0001) and the Buschke long-term recall (R2=0.08, P<0.01) accounted for significant parts of the variance.
Study 2: Autobiographical Memory
Demographic Findings:
This study included a group of 25 AD patients and a group of 20 normal individuals (10 control subjects assessed for the pilot AMS and a new group of 10 control individuals). There were no significant differences between AD patients and normal control subjects in gender (% females: 61% and 50%, respectively), and years of education (mean=12.4±4.2 and 11.0±3.6, respectively). The control group was older than the AD group (mean=64.8±9.1 years and 72.4±7.2, respectively; t=–3.6, P<0.05). The mean MMSE score for the entire AD group was 22±5.2 (range 11–30).
Autobiographical Memory Scale:
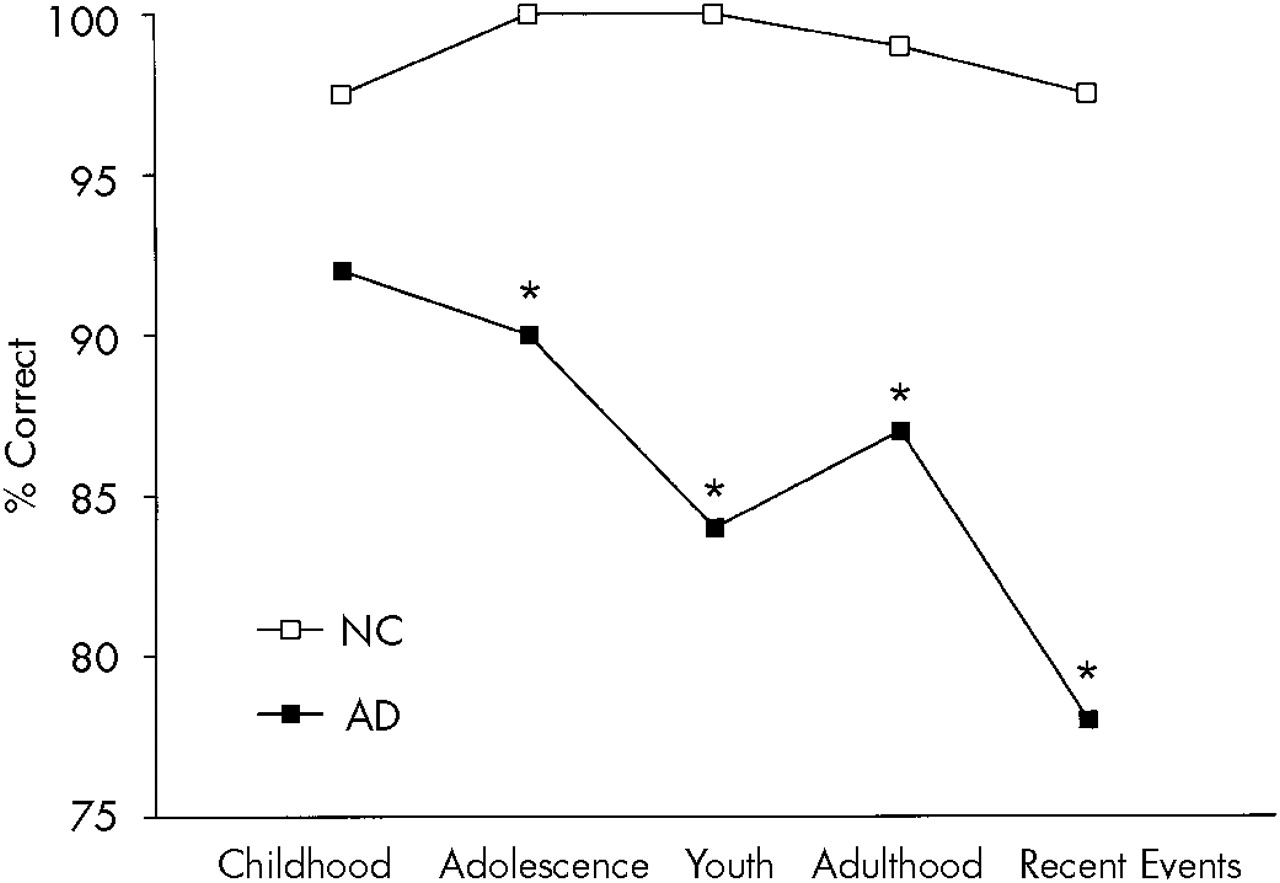
A two-way ANOVA with repeated measures for the AMS free recall (group [AD vs. control subjects]×time [childhood, adolescence, youth, adulthood, and recent events]) showed a significant group effect (
F=16.0, df=1,53,
P<0.001): AD patients had a significantly worse overall performance than normal control subjects; a significant time effect (
F=4.47, df=4,212,
P<0.001): early periods were better recalled than later ones; and a significant group×time interaction (
F=3.77, df=4,212,
P<0.005): AD patients showed significantly lower scores than normal control subjects in the recall of adolescence (
P<0.001), youth (
P<0.001), adulthood (
P<0.001), and recent events (
P<0.01), but not childhood events. AMS free recall scores for the AD group and the control group are shown in
Figure 4.
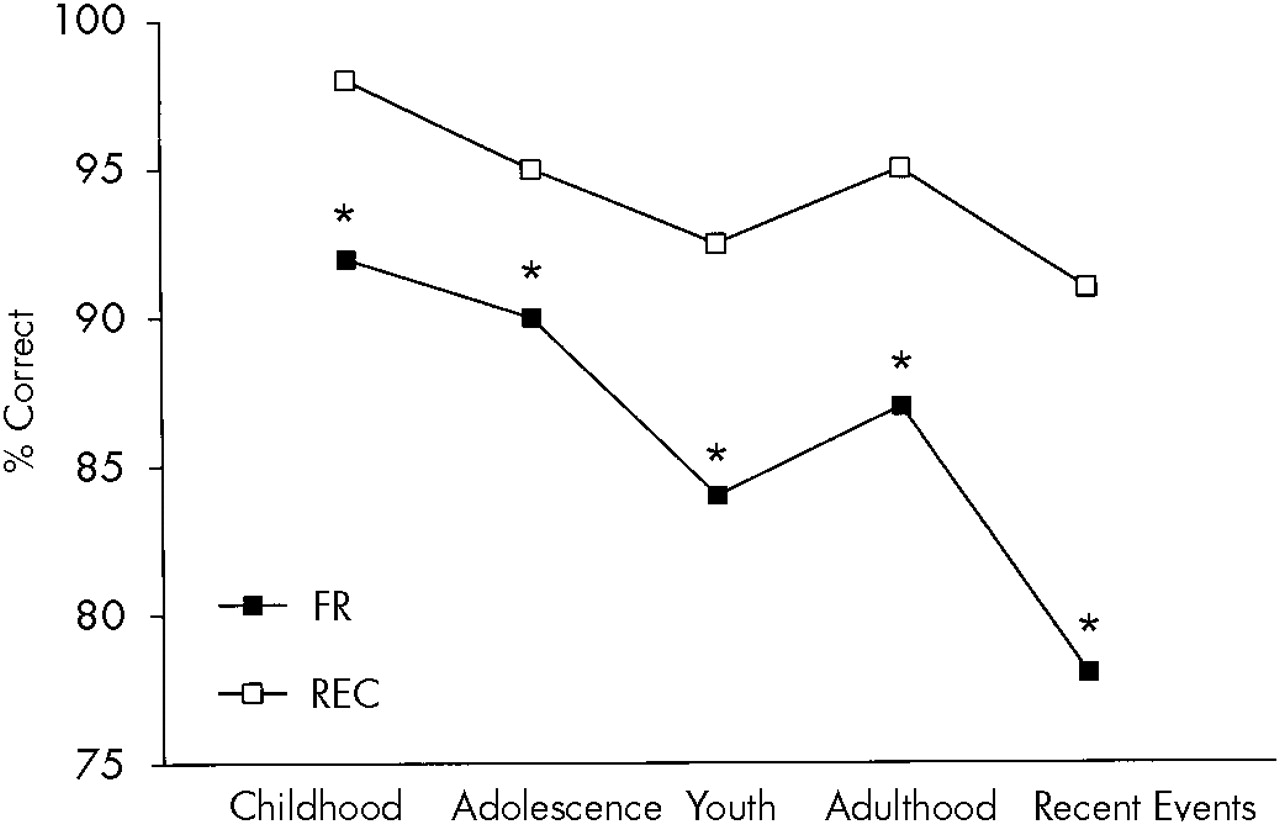
A two-way ANOVA with repeated measures for the two sections of the AMS for the AD group showed a significant test effect (
F=11.7, df=1,24,
P<0.01), with patients scoring significantly higher on the multiple choice section than the recall section, and a significant time effect (
F=8.85, df=4,96,
P<0.0001), with lower recall for adolescence (
P<0.05), youth (
P<0.0001), adulthood (
P<0.0001), and recent events (
P<0.0001) compared with childhood. Free recall and recognition scores for the AD group are shown in
Figure 5.
Neuropsychological Correlates of Autobiographical Memory in AD:
To examine the neuropsychological mechanisms that may be involved in the recall of autobiographical facts, a forward stepwise regression analysis was conducted with AMS scores as the dependent criterion variable and the neuropsychological scores as the independent predictor variables. A significant regression model was found (R2=0.77, F=12.3, df=6,23, P<0.0001), and both the Boston Naming Test (R2=0.57, P<0.0001) and the Token Test (R2=0.06, P<0.05) entered the model.
Correlation Between AMS and RMS Scores:
Twenty AD patients were examined with both remote memory scales. Whereas there was a significant correlation between RMS and AMS scores (r=0.42, P<0.05), a forward stepwise regression analysis with AMS scores as the dependent variable and the RMS and neuropsychological scores as the independent variables showed a significant correlation only with the Boston Naming Test (R2=0.52, P<0.0001); the RMS did not explain a significant part of the variance.
DISCUSSION
This study examined the presence of deficits in remote memory in patients with AD by using newly designed scales for autobiographical memory and memory for famous people and well-known events. There were several important findings. First, even patients with very mild AD had significantly more severe deficits in memory for public events (RMS) than age-comparable normal control subjects. Deficits were similar across decades, and no significant gradient was found. Second, AD patients had significantly more severe deficits in autobiographic memory than age-comparable normal control subjects. A significant gradient was found, with AD patients having significantly better recall of early memories than more recent ones. Third, AD patients showed a significantly better performance on the recognition section of the RMS compared with the free recall, suggesting that retrieval deficits and damage to memory traces may both play an important role in the impairment of remote memory in AD. Fourth, deficits in both types of remote memory were significantly correlated with deficits in verbal naming.
Our study has some methodological improvements compared with previous studies of remote memory in AD. First, we developed a test for remote memory that fulfilled critical criteria
21 such as including four time periods, showing a temporal gradient (i.e., young normal subjects remembered significantly fewer names or events from decades when they were too young to have experienced them as compared to older normal subjects), and including both a free recall and a recognition section. Moreover, we assessed a relatively large sample of AD patients and age-comparable normal control subjects, and all of the AD patients were also examined with a comprehensive neuropsychological battery. We found that AD patients had significantly more severe remote memory deficits than age-comparable normal control subjects, and these deficits were observed on both the free recall and the recognition sections of the RMS. Moreover, these deficits were already present in patients with very mild dementia. Similar to Sagar et al.
2 and Greene and Hodges,
10 we found a significant association between more severe impairments on the RMS and more severe dementia.
Sagar et al.
2 suggested that the early stages of AD are characterized by deficits in episodic memory, which may produce a temporally extensive remote memory deficit with gradient. They further suggested that as dementia progresses and semantic amnesia becomes more prominent, deficits of remote memory may become more severe, with an equal involvement of all decades. To test this hypothesis we divided our AD sample into groups with very mild, mild, and moderate dementia. Although we found a significant group effect (i.e., patients with moderate dementia had a significantly worse performance than patients with very mild dementia), there was no significant group-by-decade interaction, demonstrating that deficits in remote memory were similar for all four decades. Thus, we could not find a significant gradient for remote memory deficits in the early stages of AD in the free recall or the recognition sections of the RMS. We also found a significant (albeit low) correlation between performance on the RMS and a test of anterograde verbal memory. Whether the disruption of a common mechanism may underlie the deficits in both remote and anterograde memory in patients with AD should be examined in future studies (e.g., through a longitudinal examination of the same group of patients at different stages of the illness). There was also a significant correlation with a naming task, suggesting that remote memory deficits in AD may be partially explained by impairments of lexical retrieval.
Kopelman
4 examined autobiographical memory in AD and found a significant gradient (relatively better preservation of early versus recent memories) in both free recall and recognition tasks. Greene et al.
9 also found a temporal gradient for the incident component of the Autobiographic Memory Inventory. Our AD patients showed significantly more severe impairments than age-comparable normal control subjects on questions about adolescence, early adulthood, adulthood, and recent events, but not on questions about childhood. However, the decline in autobiographical memory was not graded; recall dropped abruptly after childhood. On the other hand, we could not find a significant gradient for the recall of famous people and well-known events, and this discrepancy may be related to differing complexity levels between RMS and AMS.
Weiskrantz
22 suggested that improvements of biographical memory on recognition testing as compared to free recall may indicate a retrieval deficit, and Dall'Ora et al.
8 suggested that autobiographical memory deficits may result from defective organization of search. We found significantly better performance in our AD patients in the recognition condition of the AMS as compared to free recall, suggesting that retrieval deficits may play an important role in the autobiographical memory deficits of AD patients. We could not compare AD and control subjects on the recognition condition because control subjects scored at a ceiling level.
An important though rarely studied issue is the association between autobiographical and anterograde memory deficits. Kopelman
4 found a significant correlation between performance on the logical memory section of the Wechsler Memory Scale (a measure of verbal anterograde memory) and scores on a scale of autobiographical incidents (
r=0.52,
P<0.05). In the present study, there was a positive correlation between AMS scores and tests of anterograde and retrograde nonautobiographical memory, but a regression analysis demonstrated that after variance in AMS performance due to naming deficits was controlled for, anterograde and remote memory deficits accounted for no additional variance in AMS scores. A similar lack of significant correlations between autobiographic memory and a test of public memory was recently reported by Greene and Hodges.
10Finally, we could not find a significant association between frontal lobe tests (such as verbal fluency and the Wisconsin Card Sorting Test) and scores on the remote memory tasks. Kopelman
11 and Greene and Hodges
6 found a significant (albeit low) correlation between frontal lobe tasks and remote memory; however, other neuropsychological tasks (e.g., language, attention, praxis) were not entered into the regression equation in their studies.
CONCLUSION
Our study presented a new scale for the measurement of remote memory, which assessed both famous names and well-known events by using a free recall and a recognition format. We found that AD patients had significant deficits in remote memory. These were already present in the stage of mild dementia and were similarly prevalent across the four decades assessed. We also designed a scale to measure biographical memory deficits in AD. Patients with AD showed significant biographical memory deficits compared with age-comparable normal control subjects, with a relatively better preservation of older than more recent memories. Future studies may examine the brain topographical correlates of biographical and nonbiographical remote amnesia in AD.
ACKNOWLEDGMENTS
This study was partially supported by grants from the Raúl Carrea Institute of Neurological Research and the Fundación Perez Companc. The authors thank Fred Bylsma, Ph.D., for his helpful suggestions.