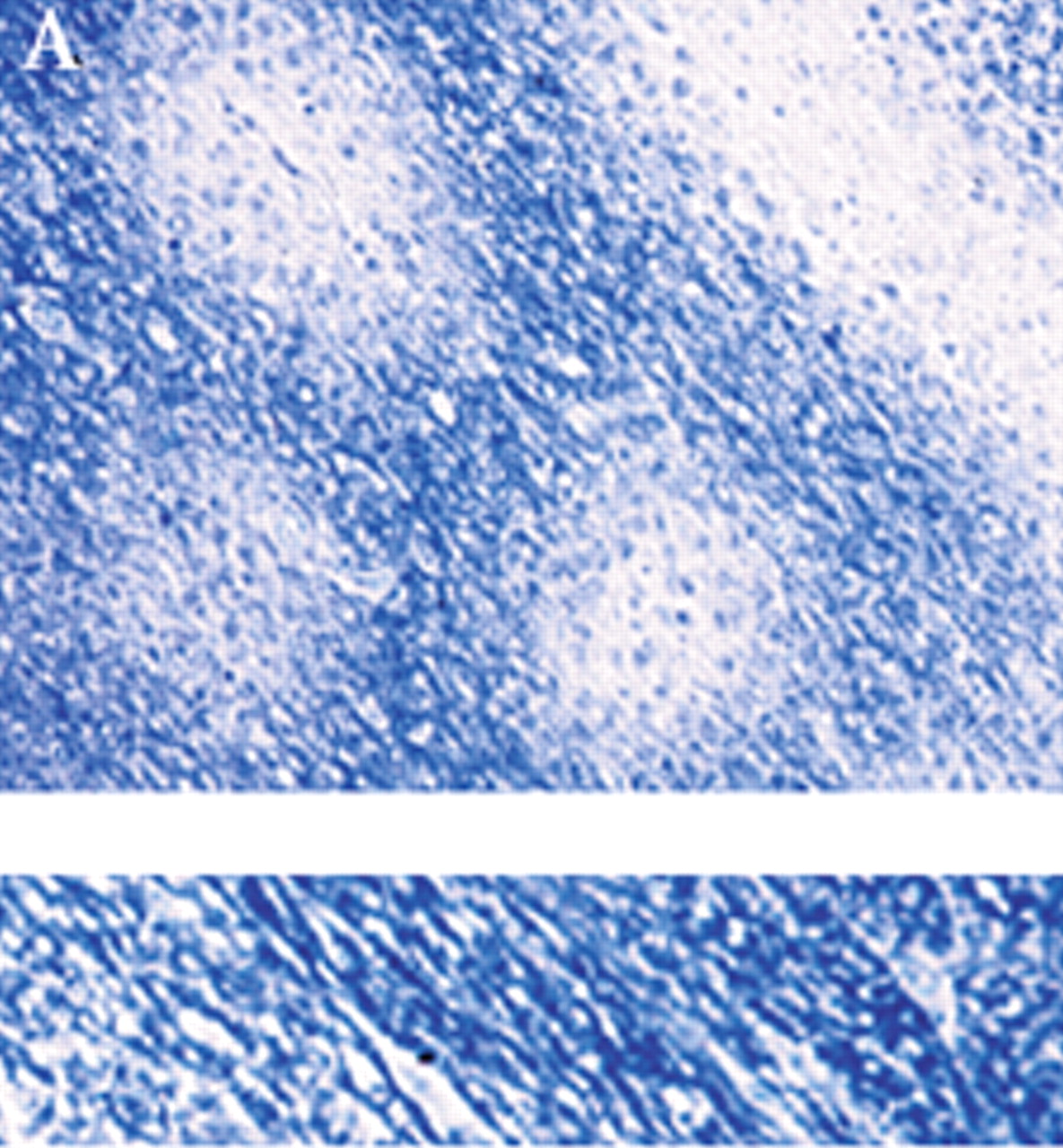
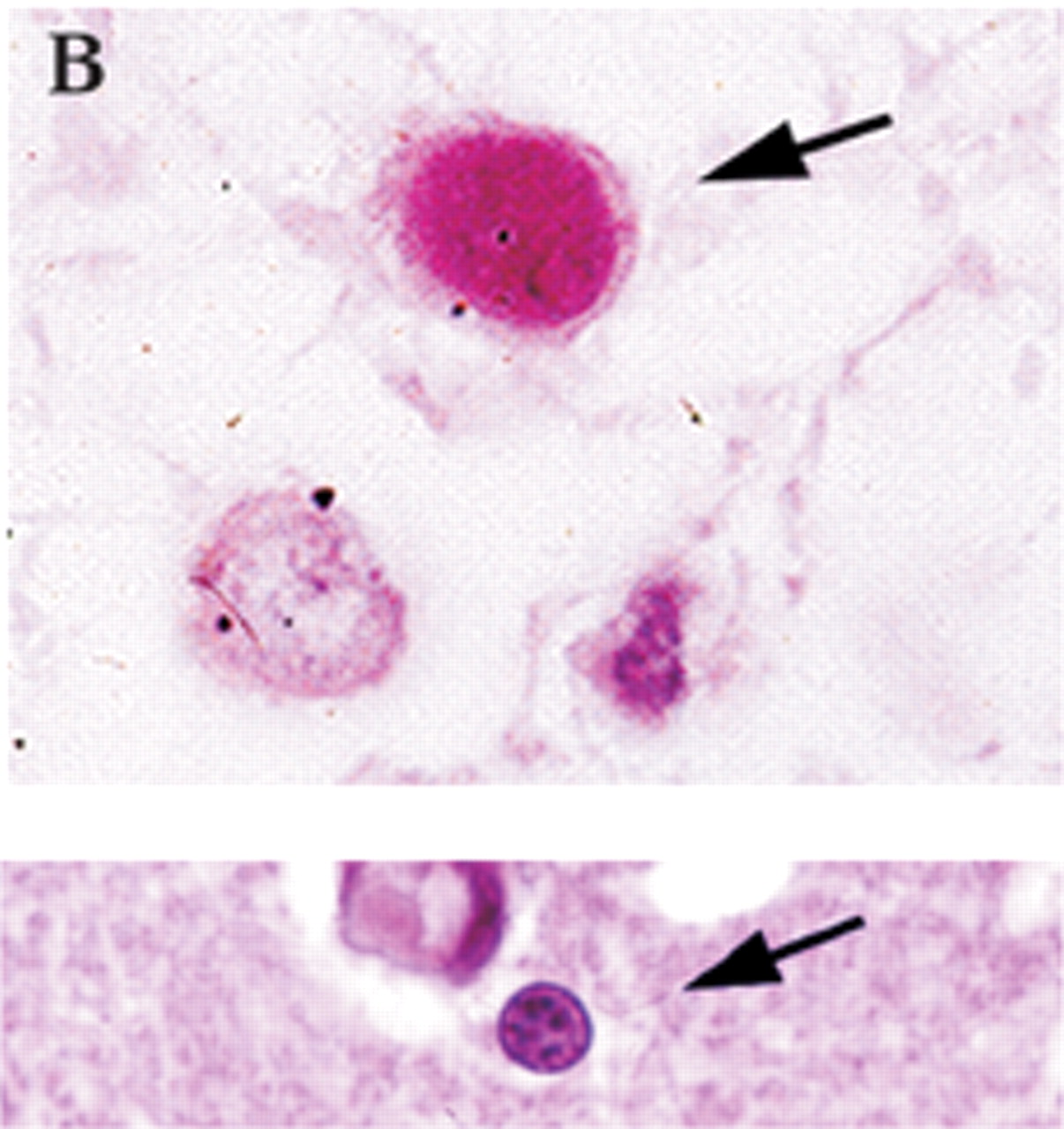
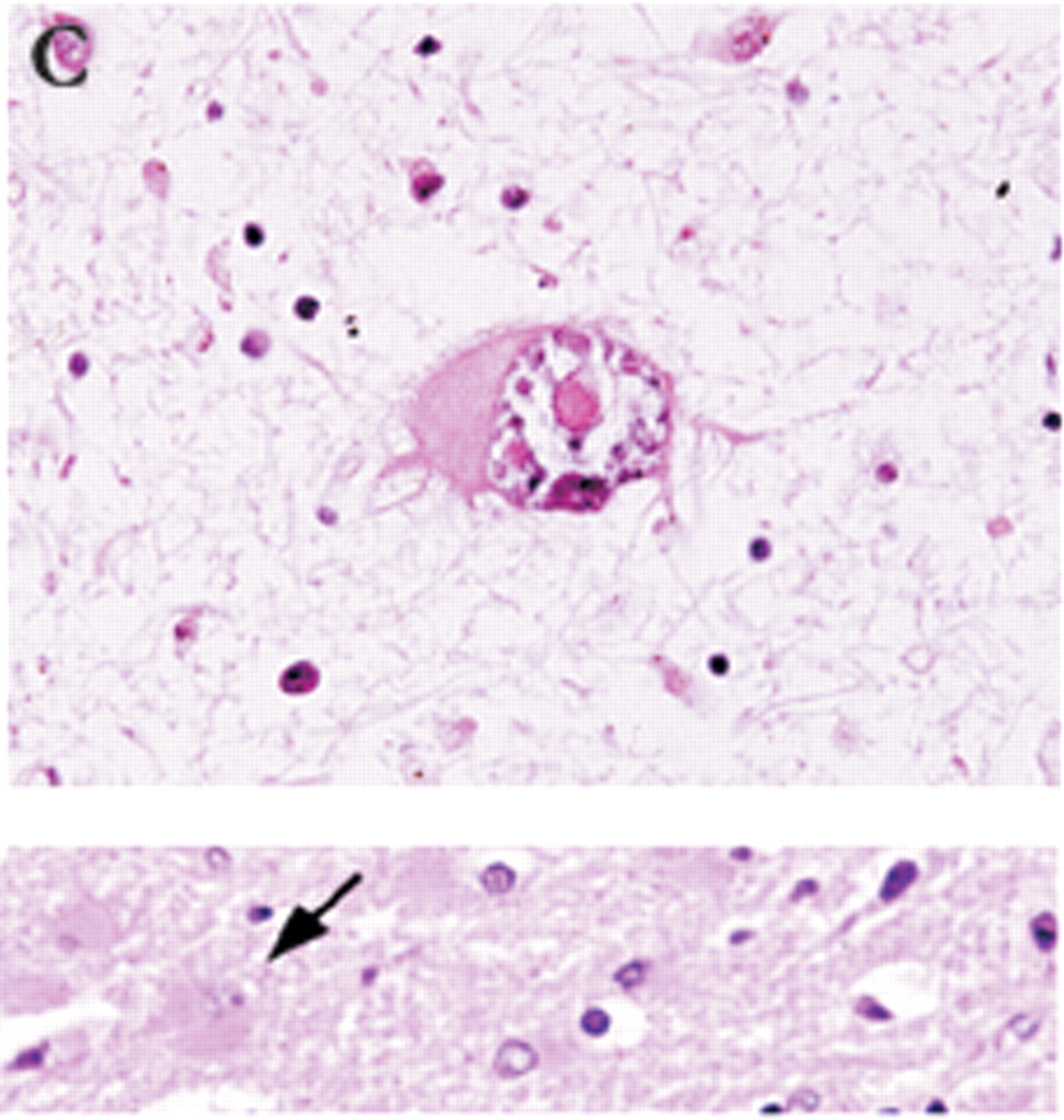
Definitive diagnosis of PML can be made only by brain biopsy. However, this is invasive and can have associated with it occasional morbidity (hemorrhage or infection within 30 days), rare mortality, or lesion of brain circuitry. In a recent review of brain biopsies of 435 patients (47 local and 388 by literature review), Skolasky et al.
13 determined that biopsies have an 88% definitive diagnostic yield. However, morbidity and mortality were high and varied from site to site. Morbidity ranged from 3.3% to 30.8% (average 8.4%); mortality ranged from 0% to 5.3% (average 2.9%). In this survey, 25% of patients were diagnosed with PML. Currently, noninvasive techniques are increasing. Radiologic imaging and CSF analysis are emerging as potential tools to replace biopsy in many cases.
Magnetic Resonance Imaging
Both PML and HIV encephalopathy are associated with nonenhancing lesions in the cerebral white matter that show little or no mass effect and are hyperintense on T
2-weighted and FLAIR magnetic resonance (MR) images.
8,14 However, PML can be differentiated from HIV encephalopathy because the PML lesions are commonly hypointense on T
1-weighted MR images, while in pure HIV infection, lesions are isointense. Serial imaging indicates the PML lesions become more hypointense as the disease progresses; markedly low signal intensity may indicate particularly aggressive infection.
8 In addition, PML lesions are focal and, if bilateral, they are asymmetric. They are located predominantly in the subcortical white matter just below the cortical ribbon, involve the arcuate (U) fibers (although brainstem and cerebellar lesions are also found), and present little or no atrophy.
15 In contrast, white matter lesions in HIV encephalopathy are diffuse and symmetric and do not involve the arcuate fibers. In addition, both cortical and subcortical (particularly caudate) atrophy are common.
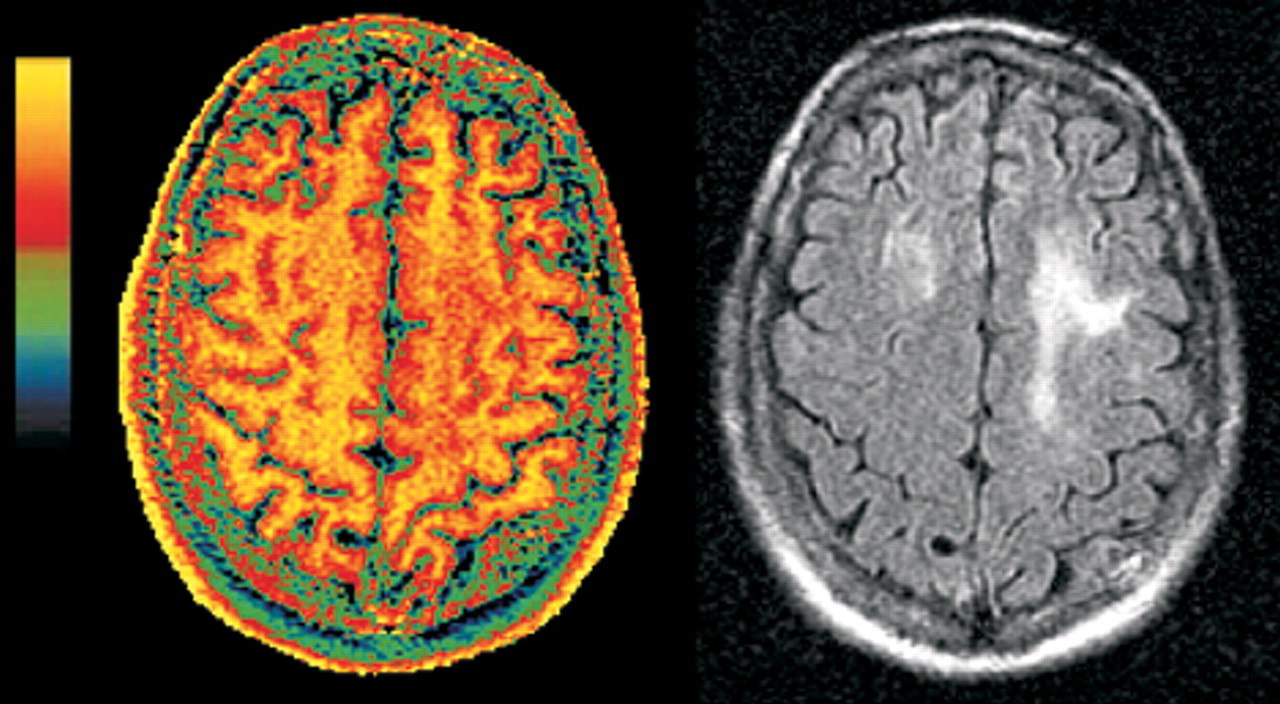
8,12,14A new type of MR imaging, magnetization transfer (MT) imaging, appears to be much more sensitive than standard MR to the demyelinating process that occurs in PML. MT imaging is based on the cross-relaxation between protons that are essentially immobile because they are bound to tissue (“solid-like” pool) and free protons (“liquid-like” pool), and thus it provides different information than is available from standard clinical MR images.
16–18 Most of the signal used to create any MR image comes from the free pool of protons. For MT imaging, specially designed saturation pulses are included in the imaging sequence to alter the magnetization of the bound protons. This in turn causes a decrease in the signal coming from the free protons and thus changes the signal intensity on the resulting MR image. Most commonly, this is expressed as the change in signal intensity relative to the normal MR image (magnetization transfer ratio, MTR, usually expressed as a percentage). The decrease in signal in the saturated image is large for complex tissues (gray matter, white matter) and small for areas that are mostly fluid (CSF, blood). The MTR is greater for white matter than for gray matter because of the high membrane content. MT imaging is sensitive to any process that disrupts the structural integrity of tissue, particularly the myelin sheath and axon.
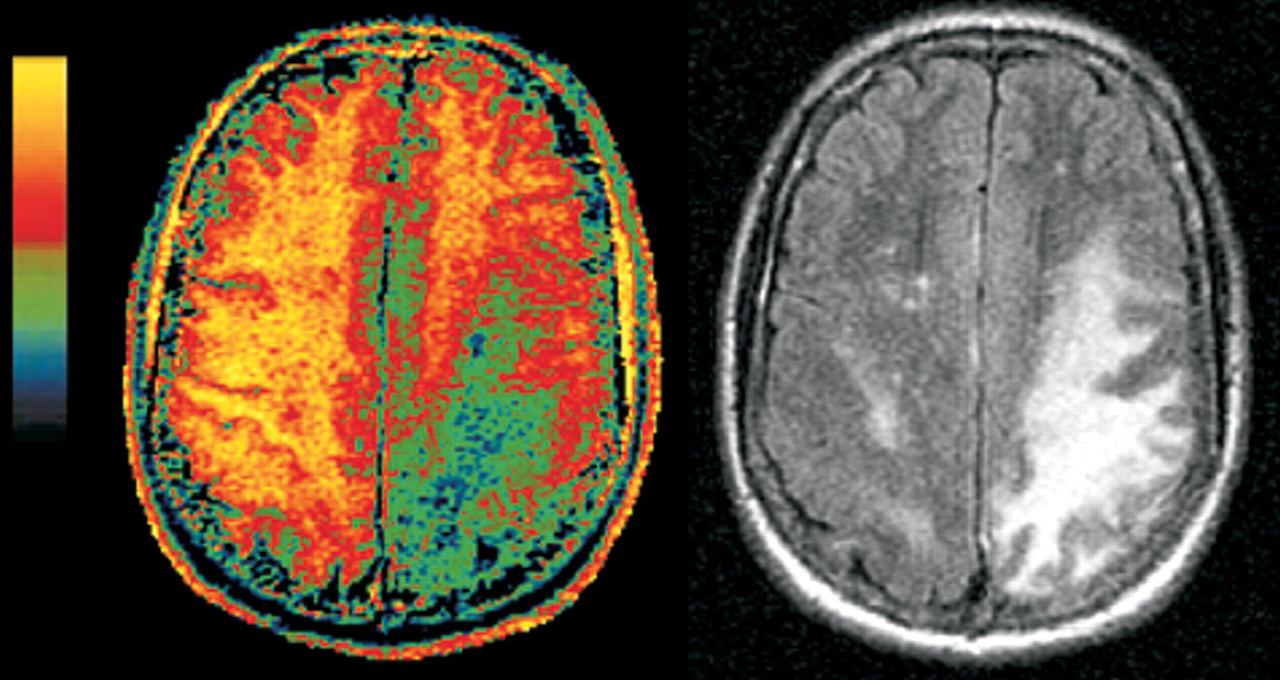
19–21Two studies have shown that MT imaging can distinguish the demyelinating lesions of PML from both the diffuse lesions of HIV encephalopathy and nonspecific white matter lesions that may be present (
Figure 2, left and
right).
22,23 There were differences between the two studies in both methodology and patient selection. In one study, the pure HIV group all had cognitive deficits, while in the other, none did. Nevertheless, the MT imaging results were similar. Both found the average MTR for normal white matter to be 47% to 48%. Both found the MTR of PML lesions to be profoundly decreased (average MTR 22% to 26%). White matter lesions related purely to HIV, with or without accompanying cognitive deficits, had mildly reduced MTR (average MTR 38% to 40%). These results indicate that MT imaging has the potential to distinguish the destructive demyelinating lesions of PML from the edematous lesions of HIV. Thus, it shows promise for differential diagnosis, prognosis, or monitoring of therapy by serial assessment.
Magnetic Resonance Spectroscopy
Proton (1H) MR spectroscopy provides measures of several neurochemicals related to neuronal viability. Signal is present from N-acetylaspartate (NAA, located primarily in neurons, a marker for neuron and axon viability), choline (Cho, principally phosphatidyl choline, a membrane constituent), and creatine (Cr, used as an internal standard because its level is usually stable). An additional peak may be present from lactate (Lac, metabolite associated with inflammation or neuronal mitochondrial dysfunction and related to activated anaerobic glycolytic metabolism). If a short echo-time acquisition is used, it is possible to observe myoinositol (MI, putative glial marker). The absolute amount of signal in each spectral peak is highly dependent on the acquisition parameters, so for comparison purposes the signals of interest (NAA, Cho) are commonly expressed relative to Cr. It is also possible to measure absolute metabolite concentrations, but the acquisition is more complex. The Cho/Cr peak reflects membrane metabolism and has been found to be elevated both during degradation (demyelination) and rapid synthesis. The NAA/Cr peak reflects neuronal and axonal density. Studies vary considerably in design and methodology, including differences in how spectra are acquired, what part of brain (lesion or normal appearing) was examined, and the types of spectral comparisons done. Nevertheless, the results are in reasonable agreement across studies.
In early HIV, when clinical symptoms are absent or mild, NAA/Cr is unchanged (92% to 98% of normal value).
24–26 In late-stage HIV, when clinical symptoms are severe, NAA/Cr decreases (62% to 84% of normal value).
24–28 Decreased NAA/Cr is found both in lesions and in normal-appearing brain.
24–30 These results are compatible with pathological findings that neuronal loss (which should result in a decrease in NAA/Cr) occurs in the late stage of HIV.
31 It is possible that in some cases decreased NAA/Cr indicates neuronal dysfunction rather than destruction. A return to normal levels was found concomitant with resolution of clinical symptoms following treatment with zidovudine in one study.
32 In contrast, Cho/Cr elevations were similar within studies in the presence and absence of symptoms and in the presence or absence of lesions on diagnostic images, although the size of the increase varied across studies.
24–26 This elevation may reflect an increase in membrane density due to glial changes and/or inflammatory cells.
24 These results suggest that the Cho peak of the spectrum may indicate the presence of subclinical HIV. MI and MI/Cr of normal-appearing white matter are also elevated in early HIV, but higher elevations are correlated with more severe clinical symptoms.
30 Thus increased MI may also indicate subclinical HIV, perhaps reflecting glial activation in response to inflammation.
Only a few studies have included patients with PML.
28,33,34 Both groups reporting on PML patients have found substantial decreases in NAA, whether measured in relation to Cr, to the contralateral NAA value, or by absolute quantification. This decrease is consistent with the destructive nature of the PML infection. Both groups have also found Cho to be elevated, perhaps reflecting myelin destruction. Lactate was present in 75% of spectra, also consistent with the severe nature of PML.
28,34 Both increases and decreases in MI were found in the one study that measured it.
33 Interestingly, the study that used absolute quantification found that Cr was decreased.
34 If this is a general finding, it will require reevaluation of studies in which ratios were used.
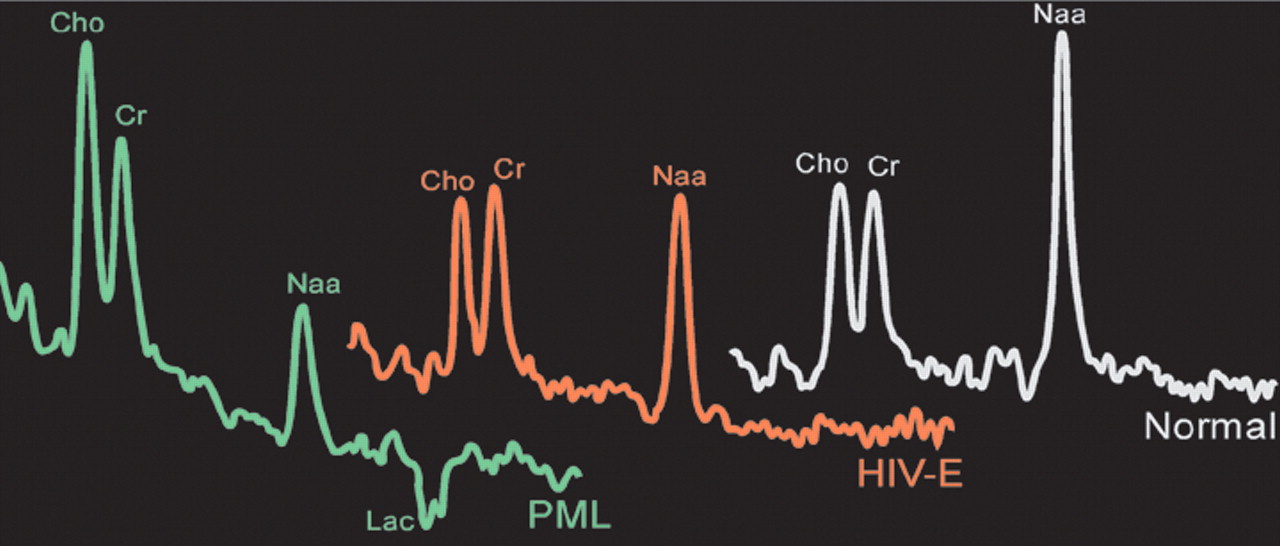
Only one study has directly compared spectral changes in pure HIV with those in PML (
Figure 3).
28 The results suggest that this technique may be helpful in differentiating PML from other entities. PML lesions had a more profound decrease in NAA than those associated with HIV encephalopathy, whereas lesions in HIV encephalopathy had a more consistent increase in Cho. In addition, PML lesions had an increased lactate peak.
CSF Analysis
JCV can be detected by polymerase chain reaction (PCR) testing of the CSF.
35 A recent review summarized the sensitivity as 90% to 100%, with a specificity of 92% to 100%.
9 However, other reports indicate that sensitivity may be lower in some circumstances.
8,23,28 PCR testing for JCV has potential as a prognostic tool because lower survival correlates with higher levels of JCV in the CSF.
9,36,37 Patients with a JCV load<4.7 log (log copies per ml of CSF) survived much longer than those with log>4.7 at time of diagnosis.
36,37 In one report, JCV load did not correlate with lesion load as measured from T
2-weighted MR images.
37 In this study of 12 patients, the volume of lesions did not correlate with JCV load even when controlled for CD4 count, treatment with HAART, plasma HIV load, age, and location of lesions. Three of the 12 patients had more than one viral load measured. Two of the three had longitudinal correlation of viral load with lesion volume, and the third was inverse. The authors recommend further longitudinal examinations in larger patient groups for any correlations with lesion volume to be found. It might also be interesting to quantify lesion load by using MT imaging.