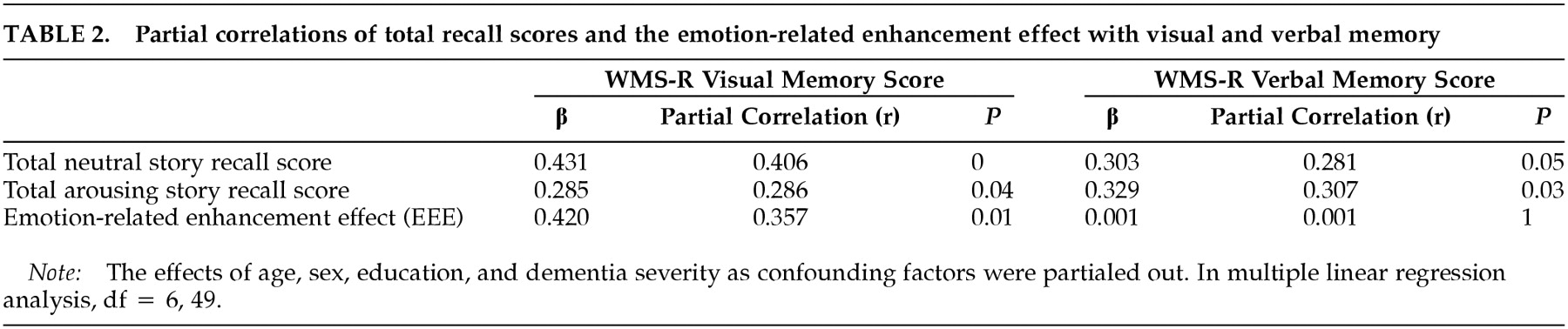
Evaluation of Effect of Emotional Arousal: The effect of emotional arousal on enhancing declarative memory was determined with the illustrated story paradigm, which has been used in a number of other studies.
3,14–17 Subjects first viewed 11 color images on a 17-inch high-resolution color monitor that were shown in sequence, accompanied by an emotionally uncharged story (the neutral story) narrative. The narrative consisted of one sentence per image and was read by a researcher (H.K.) sitting next to the subject. Together, the images and the narrative told a story. The story consisted of three phases. Phase 1 (images 1–4) depicted a mother taking her son to visit his father at work. In phase 2 (images 5–8), the boy watched a disaster drill in the hospital. In phase 3 (images 9–11), the mother was shown leaving the hospital. The images were presented on the monitor at a rate of one image per 20 seconds, controlled with the Aldus Persuasion program, version 3.0J (Adobe Inc., San Jose, Calif.) on a Power Macintosh 8100/80 (Apple Computer Inc., Cupertino, Calif.). The subjects were told to pay attention to the images and the narrative and to remember the story. Immediately after the story presentation, the subjects were asked to rate the emotional charge of the whole story on a scale of 1 to 4, with 1 indicating “not emotional” and 4 indicating “highly emotional.” Five minutes after the end of the presentation of the image, the subjects were given an 11-item recall test.
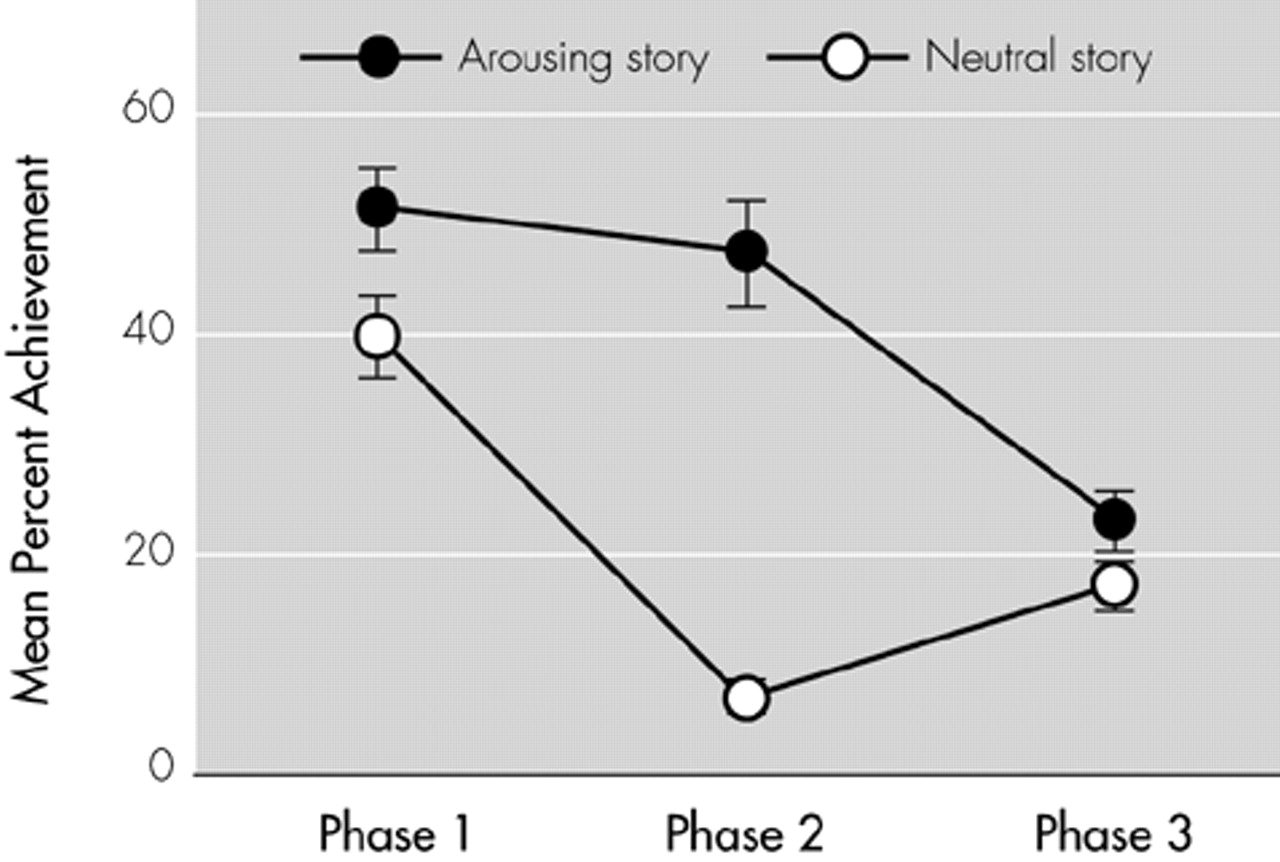
17 In the recall test, as the images were presented in the original order, subjects were asked to answer one question about the story line for each image. In the recall test, correct, partially correct, and incorrect answers received 2, 1, and 0 points, respectively. Thus, the maximum scores of phases 1, 2, and 3 were 8, 8, and 6, respectively. After an interval of 2 weeks, the experiment was repeated with the same set of images but with an emotionally charged story (the arousing story) narrative instead of the neutral story narrative. The arousing story differed from the neutral story only in phase 2, in which the boy, instead of watching a disaster drill, was badly hurt in an automobile accident, and surgeons struggled to save his life. The arousing and neutral stories are closely matched in content, complexity, and style, and they were confirmed to be matched in comprehensibility;
2 they differed in only the degree of the emotional impact of phase 2.
The trials were repeated in a fixed order after an interval of 2 weeks; with the neutral story first and then the arousing story. The reason we used this fixed-order design rather than a counterbalanced design was to avoid any different influence of the first trial on the second trial between the arousing and the neutral stories. The arousing story is assumed to have a memory-enhancing effect by emotional arousal in addition to an enhancing effect by repetition. Therefore, the influence of the arousal story presented first might be larger than that of the neutral story presented first, which would more likely affect recall performance 2 weeks later. Because we can assume that the enhancing effect by repetition is constant in all three story phases, the improved recall in phases 1 and 3 is attributable solely to the enhancing effect by repetition, whereas improved recall in phase 2 is attributable to both the enhancing effect by repetition and the effect by emotional arousal. Thus, the effect of emotional charge on declarative memory was represented by the difference of scores between the emotionally contrasted story part (phase 2), and the possible enhancing effect by repetition was canceled out by subtracting the difference of scores in the identical story parts (phases 1 and 3). Mathematically, the effect of emotional arousal on declarative memory was expressed by the emotion-related enhancement effect (EEE) index: EEE = (Ac – Nc) – [(Ai) – (Ni)], where Ac and Ai are a percent recall achievement of the emotionally contrasted story part (phase 2) and of the identical story parts (phases 1 and 3) for the arousing story, and Nc and Ni are those for the neutral story.
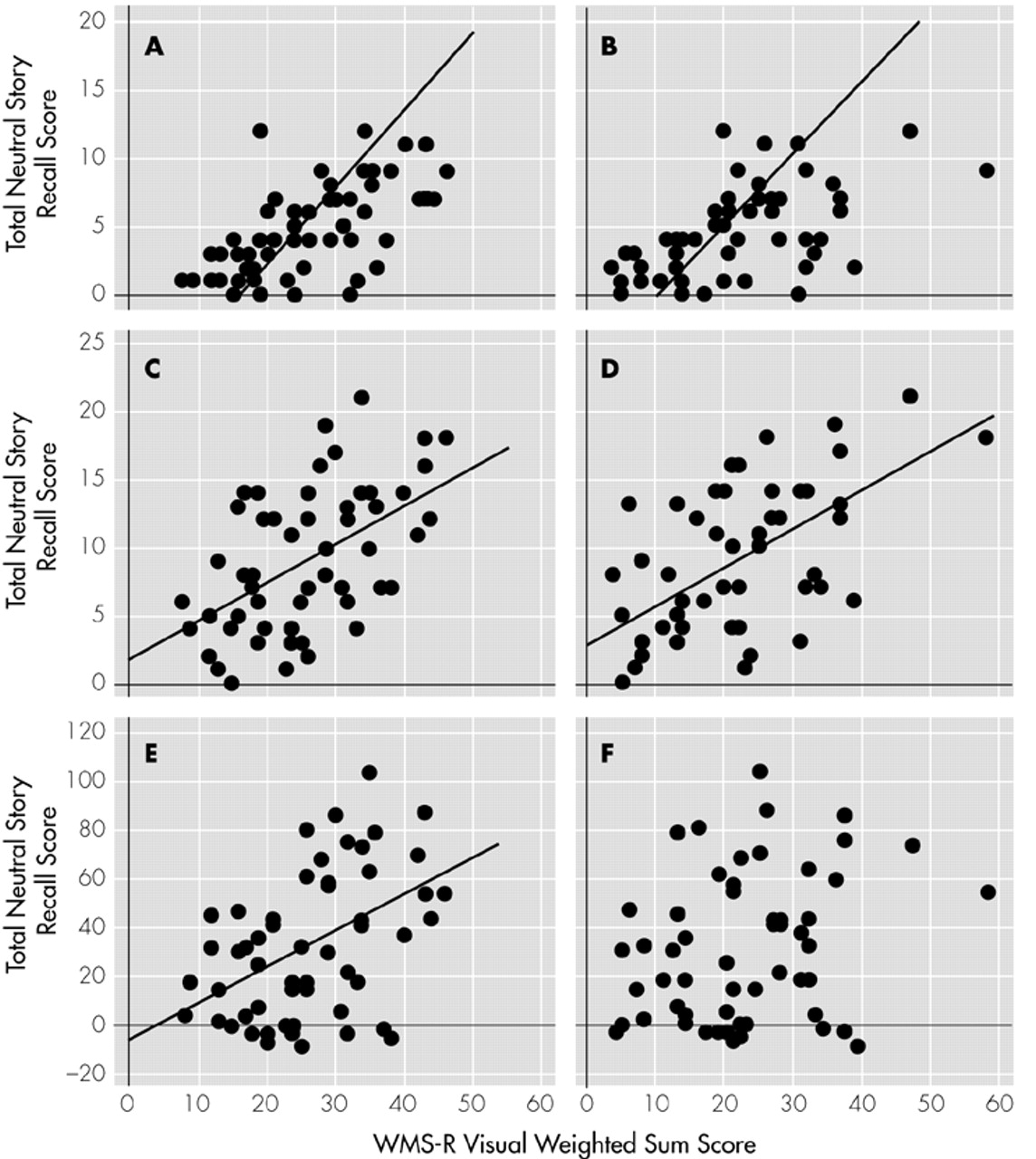
Assessment of Verbal and Visual Memory Abilities: Verbal and visual memory abilities were assessed with the Wechsler Memory Scale–Revised (WMS-R).
18 The materials in this test were ordinary and unlikely to be emotionally charged. We used the weighted sum of raw scores on the verbal and visual memory subtests for evaluations.