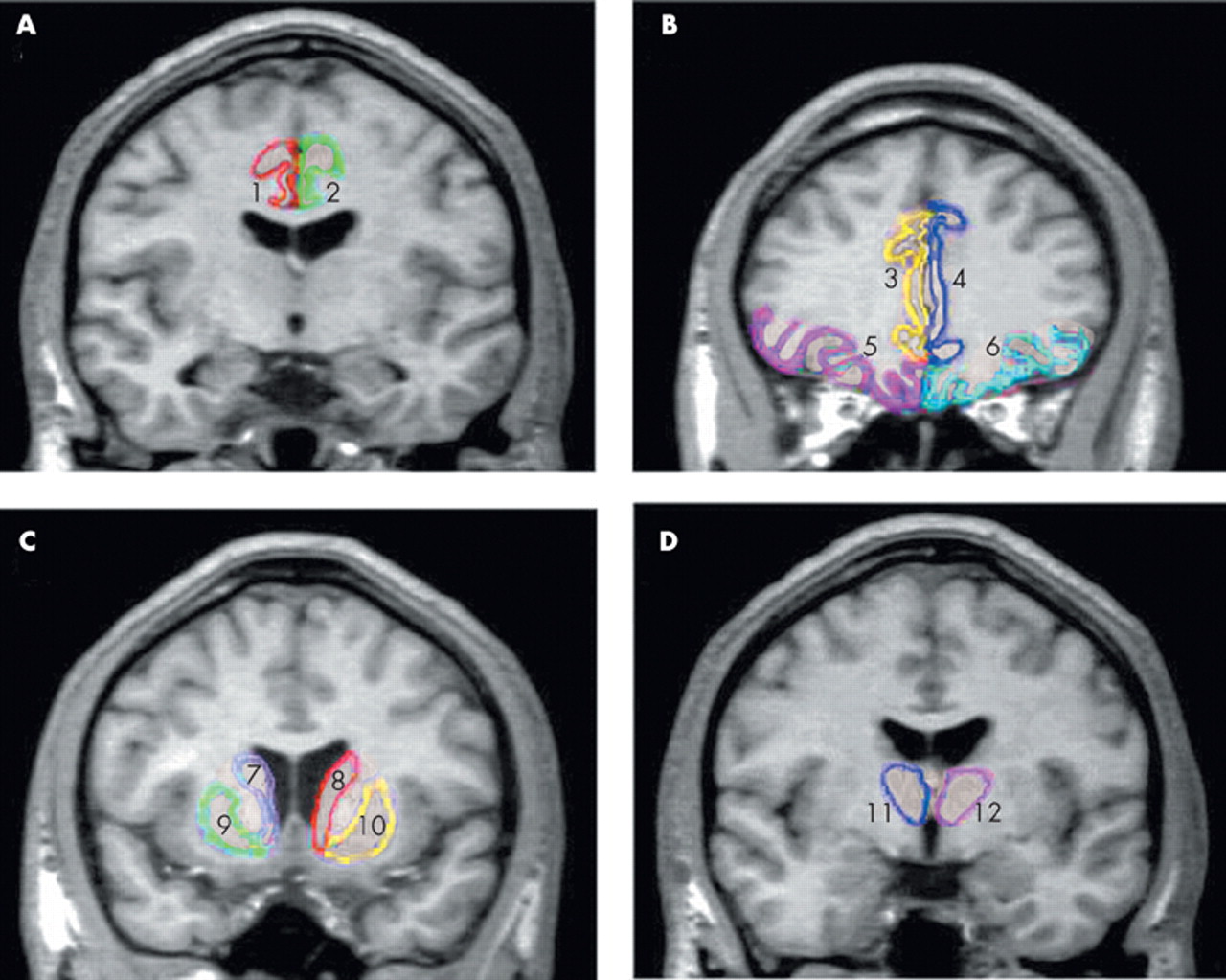
Although the pathophysiology of obsessive-compulsive disorder (OCD) remains controversial, there is substantial evidence suggesting that disturbances in the frontal-subcortical circuitry may be implicated.
1,2 Evidence of this hypothesis stems mainly from the elevated rates of OCD in individuals with basal ganglia disorders
3,4 and from functional neuroimaging studies that indicate hyperfunction in the frontal-subcortical circuitry in patients with OCD.
5–7 However, despite evidence implicating functional abnormalities in the frontal-subcortical circuitry, these regions have not been well investigated by magnetic resonance imaging (MRI). The majority of studies have examined these areas separately, and have yielded contradictory results.
8–11 Recently, considerable evidence suggests that pathological changes in patients with OCD may be expressed at the level of the spatially distributed network that subsumes the multiple, densely interconnected cortical and subcortical cortices.
12–14 This shift from key regions to key neural circuits in the research on OCD may be attributed to the diverse clinical symptoms of the disorder and neuropsychological dysfunctions observed.
Our group recently reported on the increased gray matter density of the frontal subcortical circuitry in patients with OCD by employing a novel voxel-based analysis of segmented MR images.
15 However, our finding of the increased regional density of the left orbitofrontal cortex is to some extent conflicting with previous reports by Szeszko et al.
11 In their study, Szeszko et al. reported reduced total orbital frontal volume in OCD employing the region of interest (ROI) method. In another MRI investigation using the semiautomated method for neocortical parcellation, Grachev et al.
16 reported no volume difference in the orbital frontal regions of 10 female patients with OCD and 10 female normal control subjects. Furthermore, we could not find any regional density differences in the basal ganglia structures, which are consistently suggested to be implicated in the symptomatic expression of OCD, between the patients with OCD and normal control subjects.
1,2,4,8 Although these discrepancies between studies may partly arise from methodological differences between voxel-based morphometry (VBM) and ROI methods and the heterogenous study sample, it is necessary to reconcile this disparity in order to more accurately understand the pathophysiology of OCD.
To replicate our prior VBM reports employing ROI approach, we measured volumes of the orbitofrontal cortex, anterior cingulate gyrus, thalamus, and caudate nucleus, which are the main components of the frontal subcortical circuitry in patients with OCD and in normal subjects. On the basis of our previous findings of increased regional density of the left orbitofrontal cortex and thalamus, we hypothesized that patients with OCD would also show increased gray matter volumes in those regions in which ROI method was employed.
RESULTS
Mean intracranial volume was 1,454.81 cm
3 (SD= 135.07) for the OCD group, and 1,408.98 cm
3 (SD=144.33) for the normal comparison group, which was not statistically different (
t=−1.396, df=70,
P=0.679).
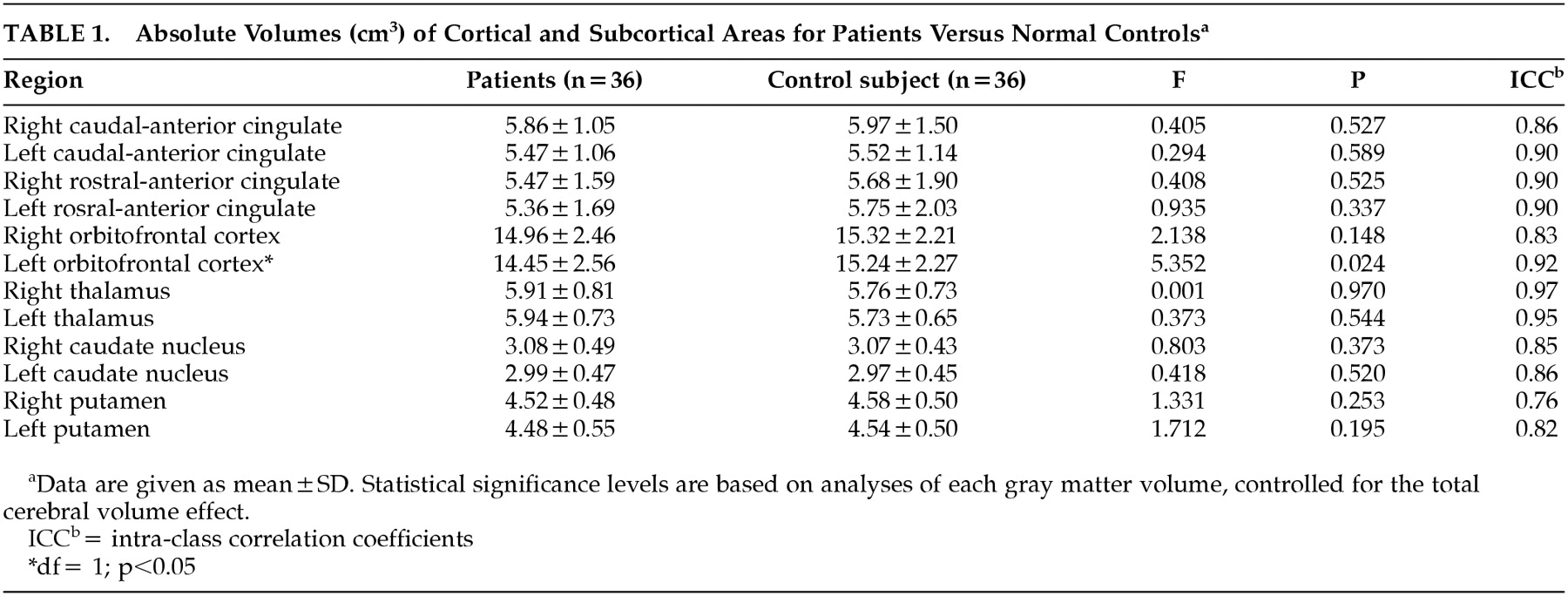
Table 1 shows gray matter volumes of each ROI subregion and the results of the ANCOVA, taking total brain volume as a covariate and diagnosis as a grouping variable. As shown in
Table 1, the left orbitofrontal volume was significantly smaller in patients with OCD (
F=5.352, df=1,
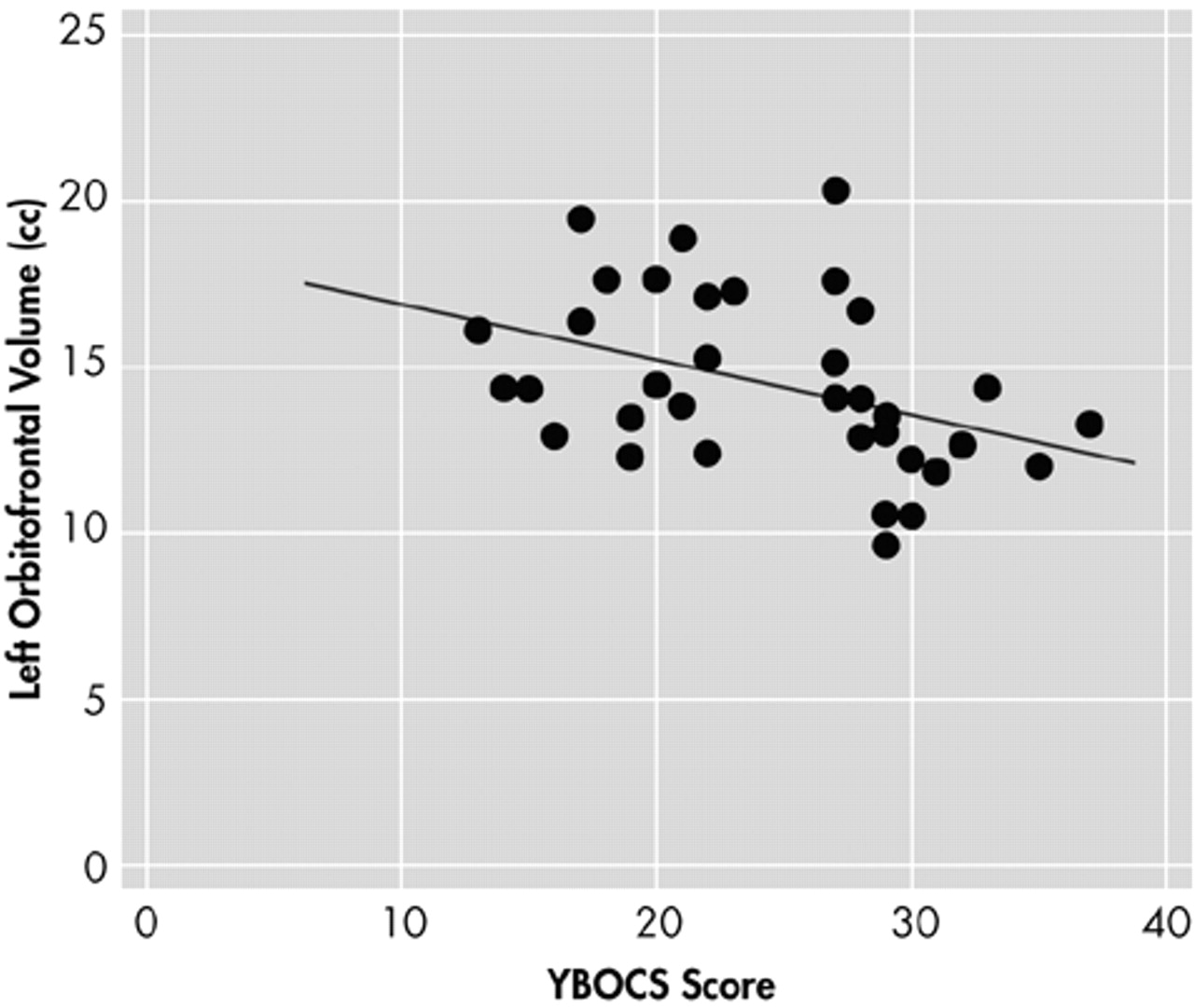
P=0.024). No significant differences were found in the volumes of the other cortical and subcortical regions measured in this study, which included the right orbitofrontal cortex, anterior cingulate gyrus, thalamus, caudate, and putamen. The volume of the left orbitofrontal cortex showed significant negative correlation (
r=−0.402,
P=0.017) with the total Yale-Brown Obsessive Compulsive Scale (Y-BOCS) scores of the OCD patients (
Figure 2), and the right orbitofrontal cortex volume negatively correlated (
r=−0.344,
P=0.040) with the compulsive subscale of Y-BOCS. However, no significant association was found between brain structure volumes and the duration of illness or age at clinical symptom onset in patients with OCD.
DISCUSSION
The main finding of this study is that the left orbitofrontal volume is reduced in OCD patients. Although there have been relatively few volumetric studies of the orbitofrontal cortex in OCD, our finding is partially consistent with a previous report by Szeszko et al.
11 In their study, Szeszko et al. reported reduced total orbital frontal volume, which included both gray and white matter in OCD, while only the left orbital frontal volume showed a significant group difference. For a parcellation of the orbitofrontal cortex, however, Szeszko et al.
11 employed topographic methods based on surface sulcal landmarks, which involve the substantial variability of the interindividual and interhemispheric gyral and sulcal patterns.
23,27 Yet, a more detailed topographic method was used in our study for a delineation of the orbitofrontal cortex, as suggested by Crespo-Faccoro et al.,
23 to reflect interindividual variability of the orbitofrontal cortex. In another MRI investigation using semiautomated method for neocortical parcellation, Grachev et al.
16 reported no volume difference in the orbital frontal regions of 10 female patients with OCD and 10 female normal control subjects. However, the statistical capacity of their study may have been limited by their small sample size. In the current study, reduced volume of the left orbitofrontal cortex in OCD was shown, using a more detailed topographic method for a delineation of the orbitofrontal cortex.
However, our finding of reduced volume of the left orbitofrontal cortex conflicts with that of our prior study, which employed VBM in OCD.
15 In the previous study, we reported increased gray matter density of the left orbitofrontal cortex. There are several hypotheses accounting for these discrepant results. First, the regional differences detected in the previous study should be viewed as representing foci of maximal change rather than regions that are exclusively affected because VBM does not reflect changes of the whole region of the orbitofrontal structure. Second, the sources of differences may arise from the changes in the shape or displacement of structures in the course of spatial normalization.
28 Finally, statistical problems of multiple comparisons may cause the different findings between studies, although we attempted to apply a strict criterion (clusters of more than 50 contiguous voxels with a threshold of
P<0.001) to avoid type I errors in the VBM study. In particular, Kubichi et al.
28 emphasized the importance of comparisons with manual ROI studies as well as careful interpretation of the results, given that factors other than volume alone influence VBM results.
In the present study, a significant negative correlation was found between the left orbitofrontal volume and the symptom severity, as measured by Y-BOCS in patients with OCD, but no significant correlations were found between the gray matter volumes demarcated and the duration of illness or the age at onset. In the previous functional studies, it was reported that the orbitofrontal cortex had increased glucose metabolic rates when compared to normal control subjects in resting states
7,29 and decreased rates after successful treatment of symptoms .
5,30 This suggests that the orbitofrontal cortex region may be involved in mediating the expression of obsessive-compulsive symptoms. Furthermore, there has been much experimental and clinical evidence that the orbitofrontal cortex is involved in the mediation of emotional response to biologically significant stimuli and in the inhibition of behavioral response.
31 Our results suggest that a structural abnormality of the left orbitofrontal cortex plays a critical role in symptomatic expression of OCD.
On the other hand, no significant group differences were found in the anterior cingulate gyrus, thalamus, and basal ganglia structures. Prior reports of a structural investigation of the caudate nucleus are controversial.
8,9,32,33 Yet, our results are consistent with those of the previous studies, which reported normal caudate volume.
9,32,33 In a comprehensive analysis of prior structural and functional imaging studies, Aylward et al.
9 suggested that the heterogenous nature of OCD might explain the inconsistencies between studies. In particular, they insisted that structural aberrations of the caudate nucleus are more evident in patients with ventricular enlargement and the soft neurological signs that are common in childhood onset. Patients in the present study were relatively free from soft neurological signs such as tics (only five patients had tic history). The nonsignificant group differences identified in the thalamus and in the anterior cingulate are consistent with previous MRI investigations in adults with OCD, which uniformly yielded negative findings.
10,11,32The most striking finding in the present study is that only the left orbitofrontal volume was significantly reduced in patients with OCD, and there were no additional structural abnormalities in other brain regions composing the frontal-subcortical tracts (i.e., abnormalities that have been implicated in previous functional neuroimaging studies of OCD, as mentioned above). One possible explanation for this morphological and functional discrepancy might be related to the methodological limitations of delineating ROIs in the current study. These may have been large enough to prevent the detection of subtle neuronal changes, and thus a significant change in some neuronal field could have been obscured, as surrounding areas within the same ROI showed no changes. In fact, other measures such as 1H-magnetic resonance spectroscopy of
N-acetylaspartate have identified abnormalities of the thalamus,
34,35 anterior cingulate,
36 and striatum
36,37 in OCD patients.
Despite these thought-provoking results, our study has several limitations. First, it does not provide information about white matter volumes because of difficulties concerning the localization of clear anatomic landmarks that define white matter subregions, which would have helped in the interpretation of our results. Second, we parceled most of the main subregions composing the frontal-subcortical circuitry, which inevitably leads to an increase in the number of comparisons, and thus increases the risk of a type I error in the interpretation of results. To conclude, we found reduced left orbitofrontal volume in patients with OCD and significant negative correlations between the bilateral orbital frontal cortex and symptom severity by simultaneously investigating the main structures composing the frontal-subcortical circuitry. These findings suggest that a structural abnormality of the left orbitofrontal cortex plays a critical role in both the clinical and cognitive expression of OCD. Future research to determine the involvement of white matter tracts of this circuitry may shed further light on the pathophysiology of this disorder.