A paradigm requiring the recognition of a recently learned face from a novel distractor is often used to examine the integrity of memory and the underlying neural substrates in both normal and clinical populations. Human lesion studies and functional imaging studies have shown that face recognition depends on the integrity of the right fusiform gyrus,
1–5 medial temporal lobe structures,
6–14 and frontal regions.
15–18Reduced ability to recognize recently learned faces has been observed in healthy older adults.
19–23 Corresponding neural substrates have shown reduced activation in healthy older adults,
24 particularly in the hippocampus,
20 during face recognition tasks. Several studies, however, have reported
increased activation in the prefrontal cortex and increased communication between the prefrontal and the occipital cortices in older adults during such tasks.
25,26 This may indicate a compensatory mechanism so that behavioral performance is maintained at a high level.
27,28Recent reports using neuroimaging techniques have suggested that age-related reduction in neural volume in regions critical to memory, with cell loss in serotonergic and dopaminergic systems, may influence the brain’s ability to neuromodulate or change activity.
29 Several studies using functional magnetic resonance imaging (fMRI) have suggested that the ability to neuromodulate as a function of cognitive demand may be impaired in older adults. For example, in a study of young and older subjects, Grady et al.
30 assessed the effect of cognitive demand on modulation of cortical activity during the maintenance phase of a variable delay working memory test for faces. Lengthening the delay interval increased memory load demand. Young subjects were able to effectively increase activity, with increasing delay interval length within a number of areas, including the anterior prefrontal and ventral extrastriate cortex. On the other hand, activity among older subjects was invariant to changes in delay interval duration. Varied results demonstrating regionally-dependent increased or decreased load-related activation in older subjects have been reported using functional MRI during tasks requiring working memory for verbal stimuli.
31In previous studies of young subjects alone, increases in memory load have resulted in increases in activity within prefrontal cortex during continuous performance tasks
32–34 and delayed-recognition tasks.
35–37 In a recent study by Jha and McCarthy, the time-course of activity was investigated in high-resolution regions of interest analysis of the prefrontal, posterior parietal, and fusiform regions during a working memory task for faces in which the number of items to maintain was varied across trials.
37 In addition to increases in prefrontal activity with higher load, increases were observed within intraparietal sulcus and fusiform gyrus. In the current study, the methodology of Jha and McCarthy was used to investigate the impact of memory load for faces during delayed-recognition performance in a group of older adults. The purpose of this study was to determine whether increasing memory load led to: 1) increased amplitude of activity within activated regions and 2) recruitment of additional activated neural volume.
METHODS
Subjects
The Duke University Medical Center Institutional Review Board approved the study, and each subject provided written informed consent after it had been fully explained. A total of 14 volunteer subjects (eight men and six women) participated (age range 59–77 years, mean 68 years). One subject was left-handed. All subjects were screened to rule out significant neuropsychiatric disorders, including dementia. The Mini-Mental State Examination
38 scores ranged from 28 to 30, with a mean of 29.3.
Cognitive Activation Task
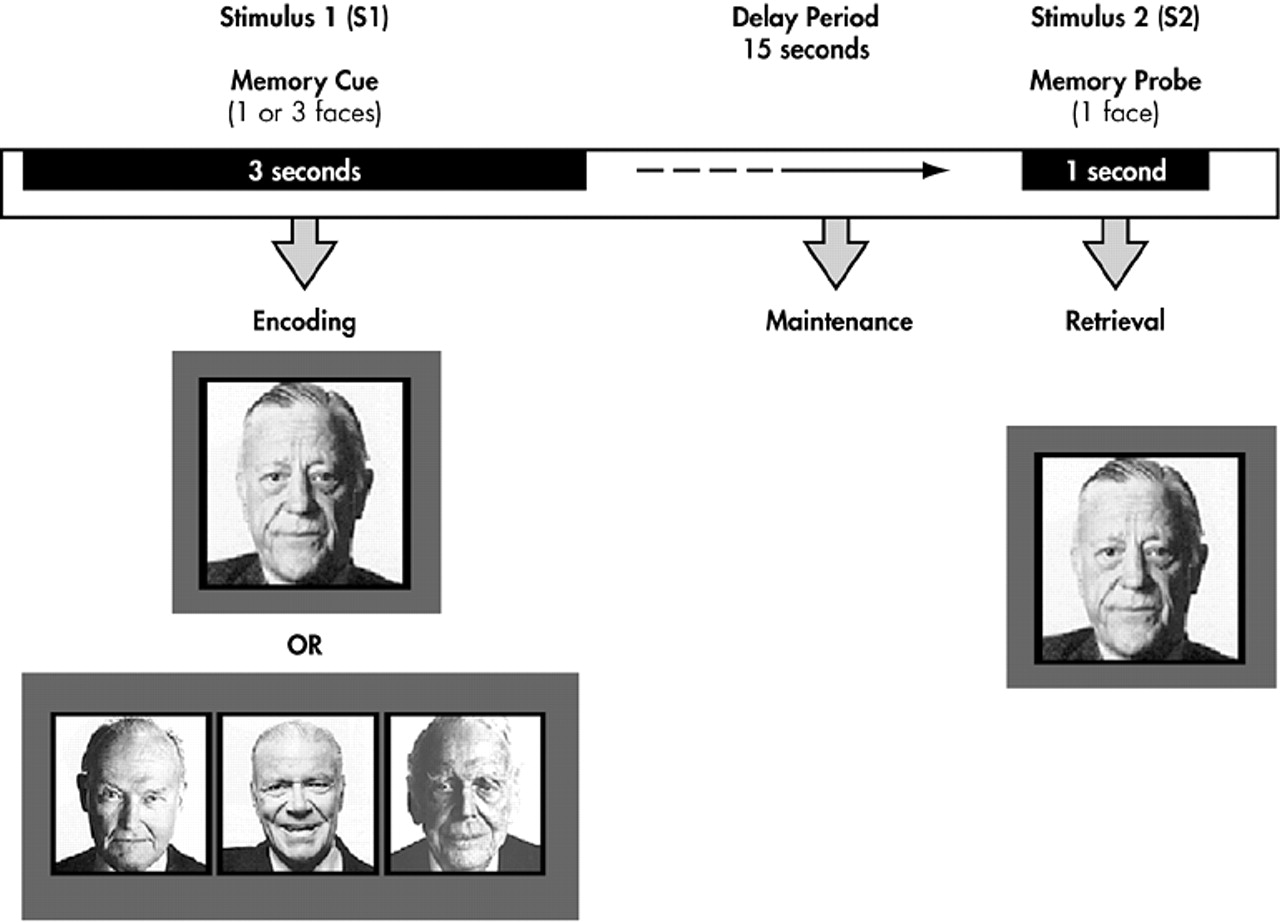
Each trial consisted of a memory array (S1) presented for 3 seconds, a 15-second-delay interval without any stimuli, and a single probe stimulus (S2) presented for 1.5 seconds that terminated the delay interval (
Figure 1). The long-delay interval was employed to disentangle transient evoked responses to S1 and S2 stimuli from delay-interval activity.
37 In one-half of the trials, the probe stimulus matched one of the items in the memory array, while in the remaining one-half the probe was a new stimulus. Subjects made a forced choice-button response with the index finger of their left or right hand to indicate whether the probe matched one of the memory items.
The memory array consisted of either one or three unfamiliar and moderately confusable faces. Faces presented in each S1-S2 trial were matched for general facial characteristics, ethnicity, gender, and luminescence. The one face and three face conditions were randomly yet equally distributed over all trials. The intertrial interval, measured from the offset of S2 to the onset of the next S1, was 29 seconds. All subjects performed six runs, with each run consisting of 6 trials (except one who only completed three runs due to an acquisition error).
All stimuli were presented using a liquid crystal display (LCD) projector (XGA resolution, 900 lumens) equipped with a specially designed Buhl lens. Stimuli were projected upon a 10-inch wide screen located within the magnet bore directly behind the subject’s head and subjects viewed the stimuli through mirrored glasses. Behavioral responses were acquired using a button box incorporating a fiber-optic loop connected to a TTL driver circuit located outside of the magnet room.
Image Acquisition
All scanning was performed on a 1.5-T NVi scanner (GE Medical Systems, Milwaukee), equipped with an Advanced Development Workstation for real time echoplanar imaging. A scout series of T1-weighted images were obtained in the sagittal plane (two-dimensional SE TR/TE=500/10, 256×192, field of view 24 cm, 5.0 mm thk/2.5 sp), followed by axial dual-echo FSE images (TR/TE 2700/20/80 256×192, field of view 24 cm, 5.0 mm thk/0.0 sp), obtained for screening purposes. A board-certified neuroradiologist (J.R.P.) evaluated all anatomical scans clinically, screening for mass lesions or other clinically significant findings. The anterior and posterior commissures were identified in the mid-sagittal slice of the initial scout series, and high-resolution coronal anatomical images were acquired from 60 contiguous 5-mm thick coronal slices using a gradient echo sequence (three-dimensional SPGR TR/TE=6/minute, 256×256, field of view 24 cm, FA=25, 5.0 mm/0.0 sp). A time series of contiguous coronal images, sensitive to blood-oxygenation-dependent contrast, were acquired from 32 contiguous coronal slice locations chosen from the three-dimensional spoiled gradient-echo (SPGR) series (GE EPI TR/TE=3000/40 ms, 24 cm field of view, 64×64 image matrix, 90° flip angle, slice thickness=5.0 mm, gap=0.0 mm), during performance of the cognitive activation task. A total of 98 brain volumes were obtained over each of the six runs described in the previous section.
Behavioral Performance
Behavioral performance results were obtained from 11 of the subjects (equipment failure precluded behavioral data collection in three subjects). Reaction time and percent correct measures were recorded. Percent correct was defined as the number of correct trials divided by the total number of trials.
Image Analysis
Preprocessing.
The center of mass for each echo planar imaging (EPI) volume within each time series was computed and plotted to determine head motion during scanning, revealing submillimeter motion in all subjects. The time series of EPI volumes was adjusted to compensate for the interleaved slice acquisition order within each repetition time (TR) interval. Fitting the time series of each voxel with a cubic spline and then resampling this function for all voxels at the onset of each TR interval accomplished this.
Processing.
Average time-epochs synchronized to S1 were computed for each memory load condition according to the methodology of Jha and McCarthy.
37 In brief, 16 image time segments, from the five image volumes preceding to 10 following each S1 stimulus, were excised from the continuous time series of volumes for each run comprising each subject’s data. These single trial volume epochs were then grouped by stimulus type (1- or three-face trials) and averaged, with the temporal order of each volume relative to S1 maintained. The baseline was calculated as the mean intensity of the two time points prior to S1. The average MR signal values were then converted to percent signal change relative to this pre-S1 baseline. The averaged time-epochs were then subjected to the analytic procedures described below.
Regions of Interest
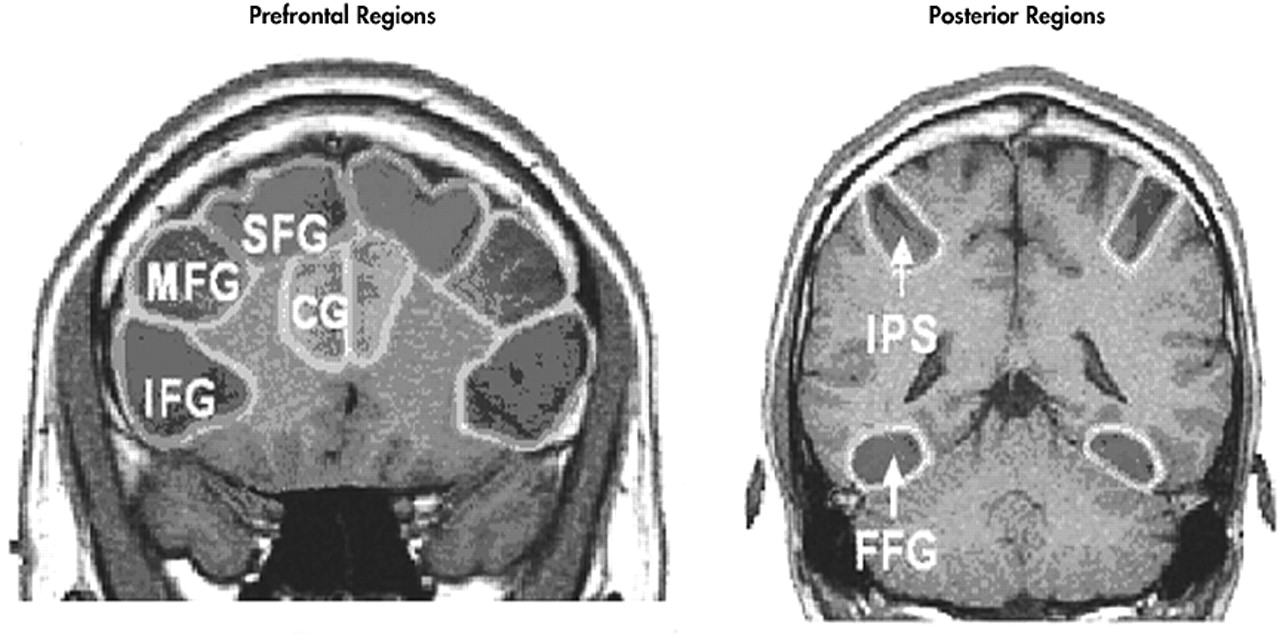
Anatomical regions of interest (ROIs) were drawn on each subject’s high-resolution coronal anatomical images. Regions of interest were drawn on the left and right superior frontal gyri, middle frontal gyri, inferior frontal gyri, anterior cingulate gyri, intraparietal sulci, and fusiform gyri. The ROIs for superior frontal gyrus, anterior cingulate gyrus, middle frontal gyrus, and inferior frontal gyrus were drawn on nine slices ranging from 35 mm anterior to 5 mm posterior to the anterior commissure. The fusiform gyrus was drawn on six slices ranging from 20 mm to 45 mm posterior to the anterior commissure. The intraparietal sulcus was drawn on eight slices ranging from 50 to 85 mm posterior to the anterior commissure (
Figure 2).
Time Course Analysis
Since the activity measured within an ROI might represent the activity of only a few activated voxels diluted by surrounding unactivated space, a subset of activated voxels were defined, and time-activation waveforms were computed for only activated voxels located within each anatomical ROI. To identify a subset of activated voxels, a correlation analysis was performed using a reference waveform, based on empirical data obtained during a similar task.
37 Active voxels were defined as those that had a waveform that significantly correlated with the reference waveform (t>1.96, p<0.05, uncorrected). By using a low statistical criterion for the significance of the correlation, we purposely defined “activated” as those voxels that had waveforms that could vary somewhat from the reference waveform. As there were differences in the number of activated voxels as a function of memory load, the time waveforms were computed for the union of active voxels for both load conditions for each ROI. Each subject contributed 24 waveforms (six anatomical regions by two hemispheres by two memory load conditions).
Activated Voxel Counts
Using the ROIs defined above, we tabulated the number of active voxels within each anatomical ROI for each load condition. These counts were converted to percentages relative to the number of voxels in each ROI to normalize for differences in brain volume across subjects.
Statistics
For comparison of behavioral responses between memory load conditions, reaction time and percent correct measures were entered into separate paired Student’s t tests. For comparison of the waveforms, repeated measures analyses of variance (ANOVA) were performed to determine if activity differed as a function of memory load at each time point in the activation time-waveforms for each ROI . Finally, for comparison of activated voxel counts, repeated measures ANOVA were performed to determine if the number of activated voxels differed across group, gyrus, hemisphere, and memory load condition. Our statistics did not include corrections for multiple tests.
DISCUSSION
Our study demonstrates that there is an increase in magnitude of brain activation in response to increased memory load in healthy older subjects. These findings suggest that such individuals may have neurofunctional reserves that can be called upon to handle more challenging memory tasks. The pattern of increased activation is highly similar to that observed in younger individuals doing a similar task in which they demonstrated increased activation in both three-face and two-face tasks compared to a one-face task.
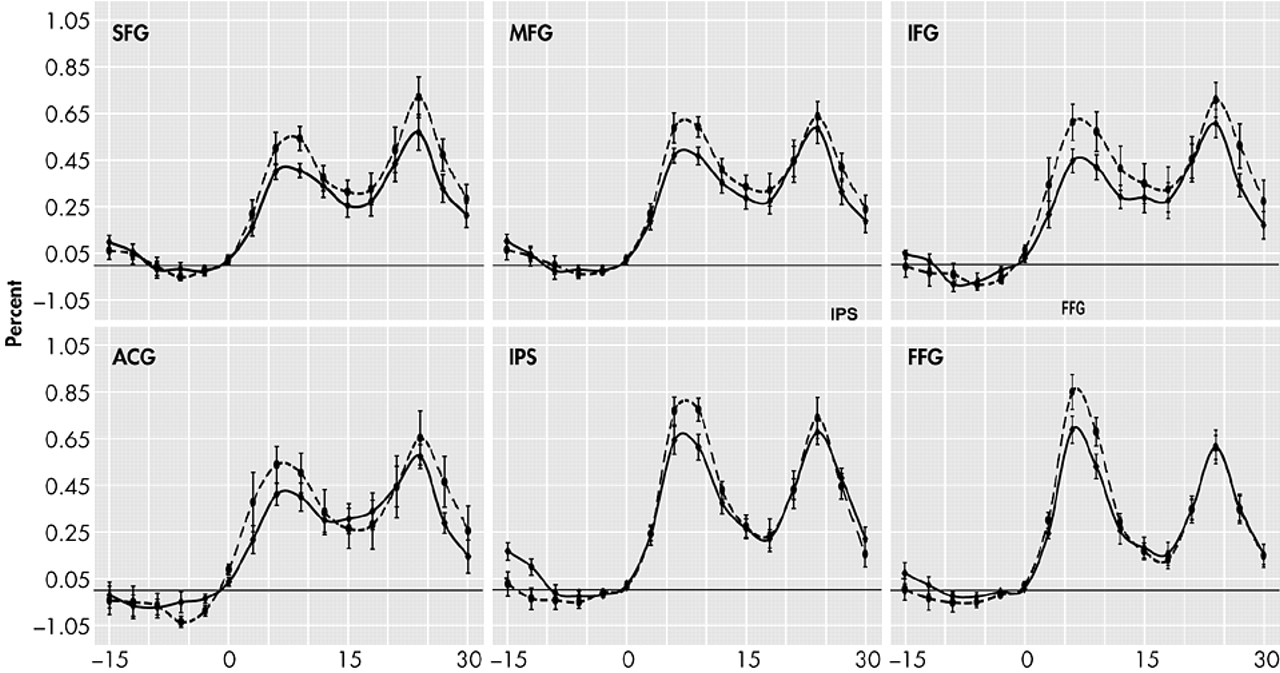
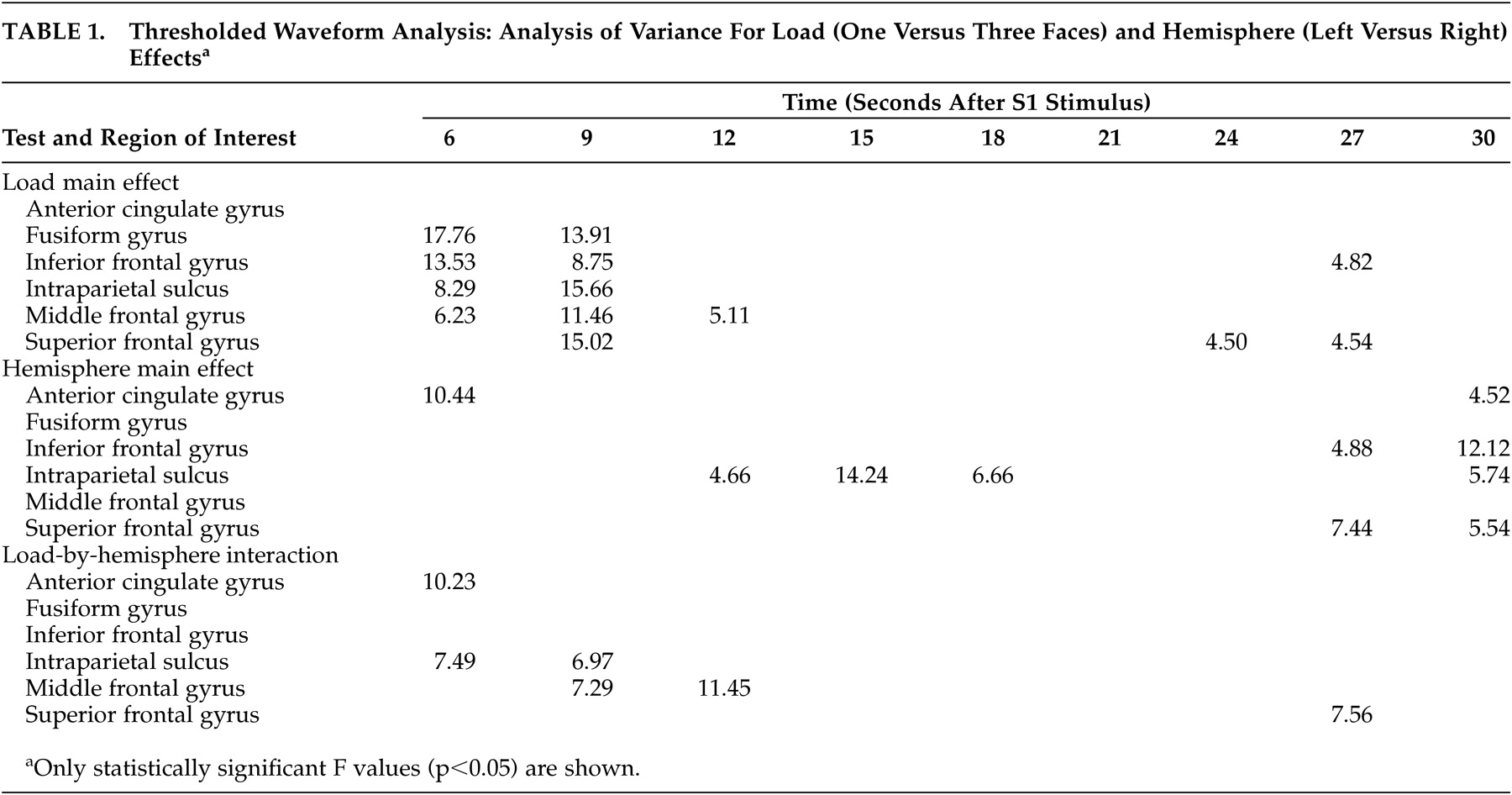
37Memory load only affected the waveform during the encoding and transition (early delay) period of our memory task. The prefrontal cortex, intraparietal sulcus, and fusiform gyrus all showed statistically significant activation differences due to memory load at the 6- and 9-second time points, but by 12 seconds, only the middle frontal gyrus still showed significant activity change. These findings are consistent with those of D’Esposito et al.,
39 who found the encoding process in older subjects was significantly affected by memory load.
None of our subjects showed load-related differences in the delay interval. These results are consistent with the patterns found in young individuals by Jha and McCarthy, who observed that while phasic processes such as encoding were affected by memory load, maintenance processes were not.
37 During the retrieval period, our results show statistically significant load-related activation differences in the superior frontal gyrus at two time points and in the inferior frontal gyrus at one time point. Prior studies have shown load sensitive retrieval effects only in young subjects.
40Our observation that the intraparietal sulcus responded similarly to the prefrontal cortex when the memory load increased replicates findings of Braver et al.,
34 who observed increased activation bilaterally in the prefrontal cortex and the parietal regions with increased memory load in a young population. Manoach et al,
41 however, found that while both dorsolateral prefrontal cortex and intraparietal sulcus were activated by memory tasks, only the dorsolateral prefrontal cortex was affected by changes in memory load.
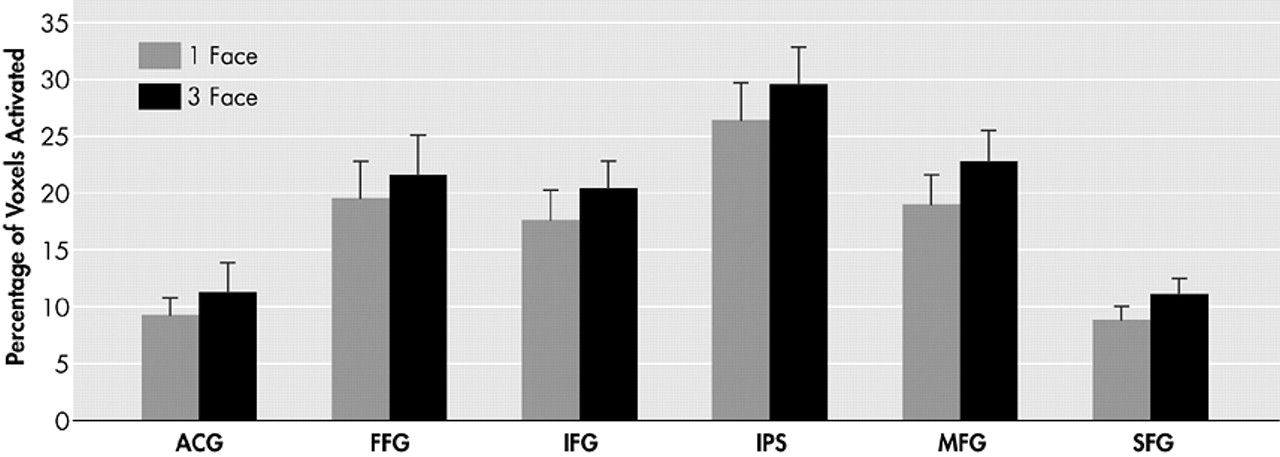
Our findings demonstrate a significant increase in the magnitude of activation in the prefrontal cortex region (inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus) in the three-face condition compared to the one-face condition. However, we were unable to demonstrate a statistically significant increase in extent of activation measured by voxel counts. Rypma et al.
31 suggested that older individuals might be limited in their ability to recruit additional dorsolateral prefrontal cortex regions for more challenging memory loads. Alhough it is possible that new regions of the prefrontal cortex cannot be recruited, and only regions already activated in the lower memory load condition may have the ability to raise their level of activation when presented with a memory load challenge, the current study suggests that this recruitment ability is indeed preserved. A clear trend toward a positive load affect on voxel count was present in every ROI and approached statistical significance when all regions were combined (
Figure 4).
Using a very similar paradigm with younger subjects, Jha and McCarthy
37 noted a statistically greater number of activated voxels in their three-face and two-face trials compared to their one-face trial. This was found in a number of the same regions tested in this study: the middle frontal gyrus, inferior frontal gyrus, anterior cingulate gyrus and intraparietal sulcus. Therefore, a functional explanation needs to be considered for the ability to increase the
magnitude, but to a lesser degree, the
extent of activation in response to increasing memory load in older adults. Age-related neuronal loss has been well documented (see reference 42). Though vascular reserve capacity may be relatively preserved in older subjects,
43 it is possible that there is less capacity to recruit additional neurons to deal with increased task load due to age related neuronal loss. A comparison of regional brain volumes was performed between this study and that of Jha and McCarthy,
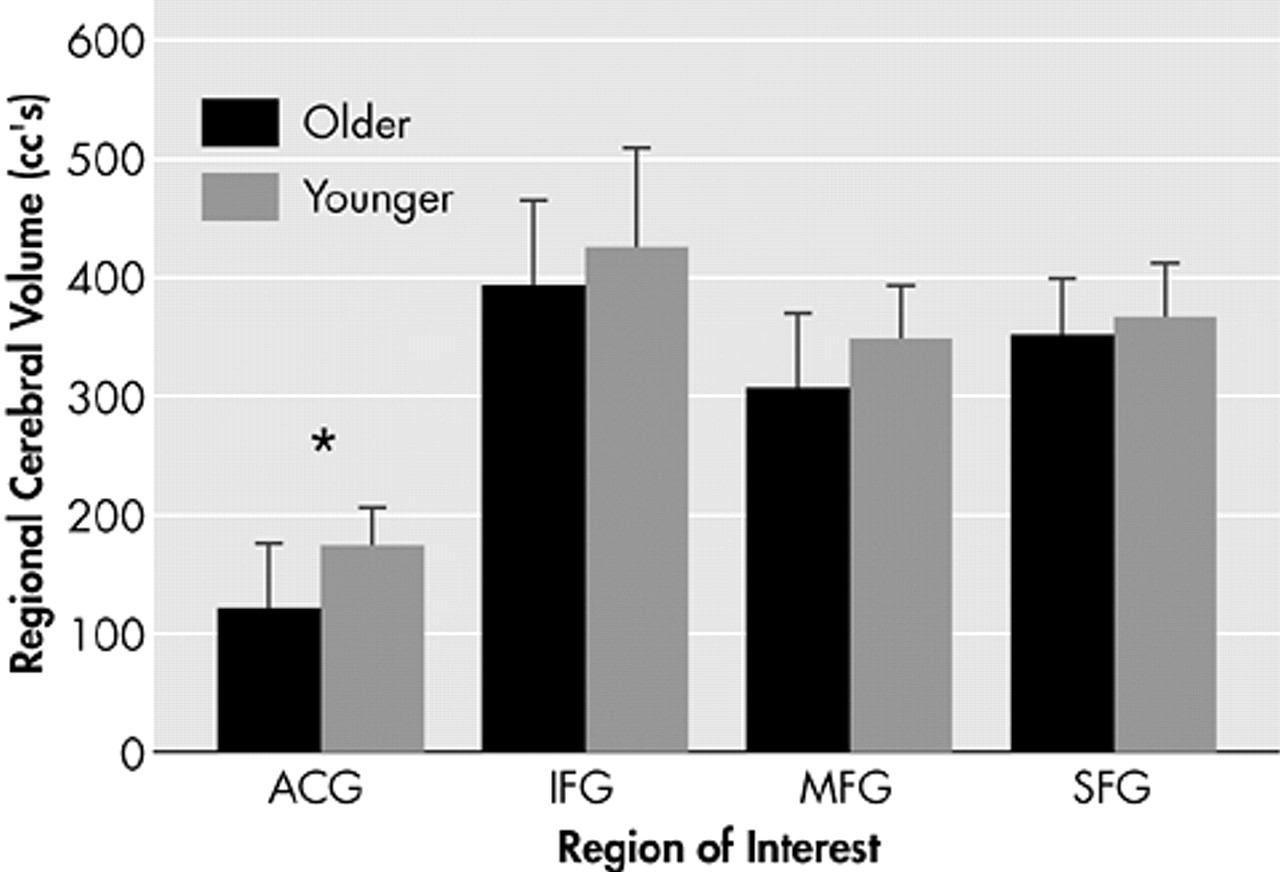
37 in regions of interest defined by identical anatomical criteria, namely the superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus and anterior cingulate gyrus. The total number of voxels in each region was tabulated for each subject in both studies and multiplied by the appropriate voxel size. Results show a trend toward decreased volume in older subjects in the prefrontal cortex, with a statistically significant decrease in the anterior cingulate gyrus (
Figure 5).
In conclusion, this study in healthy older adult subjects demonstrates similar changes to those described in young subjects, namely that increasing memory load increases the magnitude of signal change in activated voxels in memory-specific brain regions in the frontal, temporal, and parietal lobes. Load-related increases in the extent of activation are less robust, which may be a consequence of age-related volume loss with decreased capacity for neuronal recruitment.
ACKNOWLEDGMENTS
Dr. Petrella’s research is supported by grants from the Radiological Society of North America Research and Education Foundation and National Institute of Aging, 1R01 AG-019728-01. The authors thank Gregory McCarthy, Ph.D. for his advice on experimental design and analysis techniques, and Haris Sair, B.S., and Lakshmi Murty, B.S. for assistance with analysis and proofreading. Aspects of this study were presented at the Ninth Annual Meeting of the Cognitive Neuroscience Society, April 14–16, 2002.