Obsessive-compulsive disorder (OCD) is a disabling psychiatric condition characterized by recurrent and disturbing thoughts or images (obsessions) and repetitive and/or ritualistic or stereotyped behaviors that a person feels driven to perform (compulsions).
1 Although the primary pathogenesis of OCD is uncertain
2 OCD has been considered a neuropsychiatric disorder with altered neurological function.
1 According to the literature, obsessive-compulsive symptoms have been associated with different types of brain damage or dysfunction as follows. Alegret et al (2001) reported that patients with severe Parkinson’s disease had more obsessive symptoms than normal population.
3 Furthermore, obsessive-compulsive symptoms have been found in postencephalitic patients.
4 There are case reports of obsessive-compulsive disorder associated with pediatric cerebral malignancies.
5,6 In the case reports of adult patients’ feelings the onset of obsessive-compulsive symptoms has been considered to be a sign of underlying organic pathology of a primary brain tumor
7,8,9 or head trauma.
10,11 Recently Stengler-Wenzke et al.
12 suggested that secondary OCD is caused by brain injury in the fronto-orbito-striatal region,
12 and Stein (2002) reported that functional imaging studies have found altered activity in the orbitofrontal cortex and caudate, especially during exposure to feared stimuli among OCD patients.
1To our knowledge, there are no population based clinical studies assessing the obsessive and compulsive symptoms among patients with a brain tumor. Thus, it can be hypothesized that secondary OCD is strongly present among brain tumor patients who have brain dysfunction and who are at the same time afraid of the operation associated with their severe disease and exposed to fear of dying. We investigated the level of obsessionality among brain tumor patients with preoperative and postoperative measurements by taking into account also the location and histology of the tumor.
RESULTS
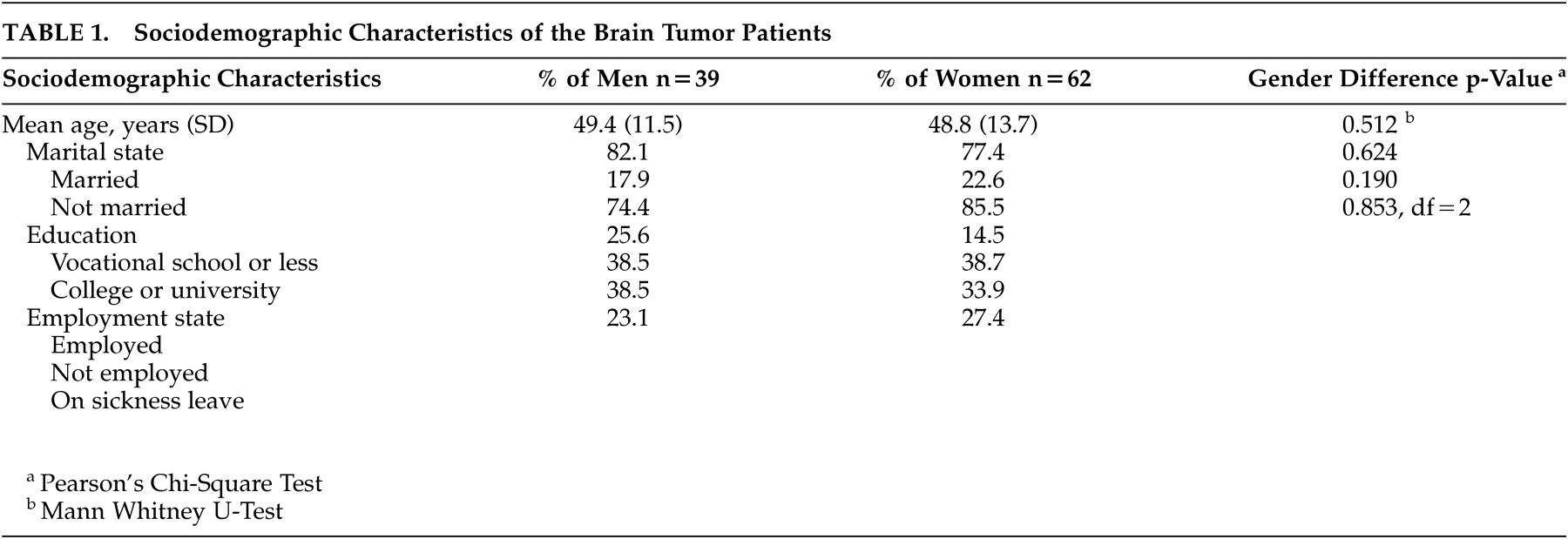
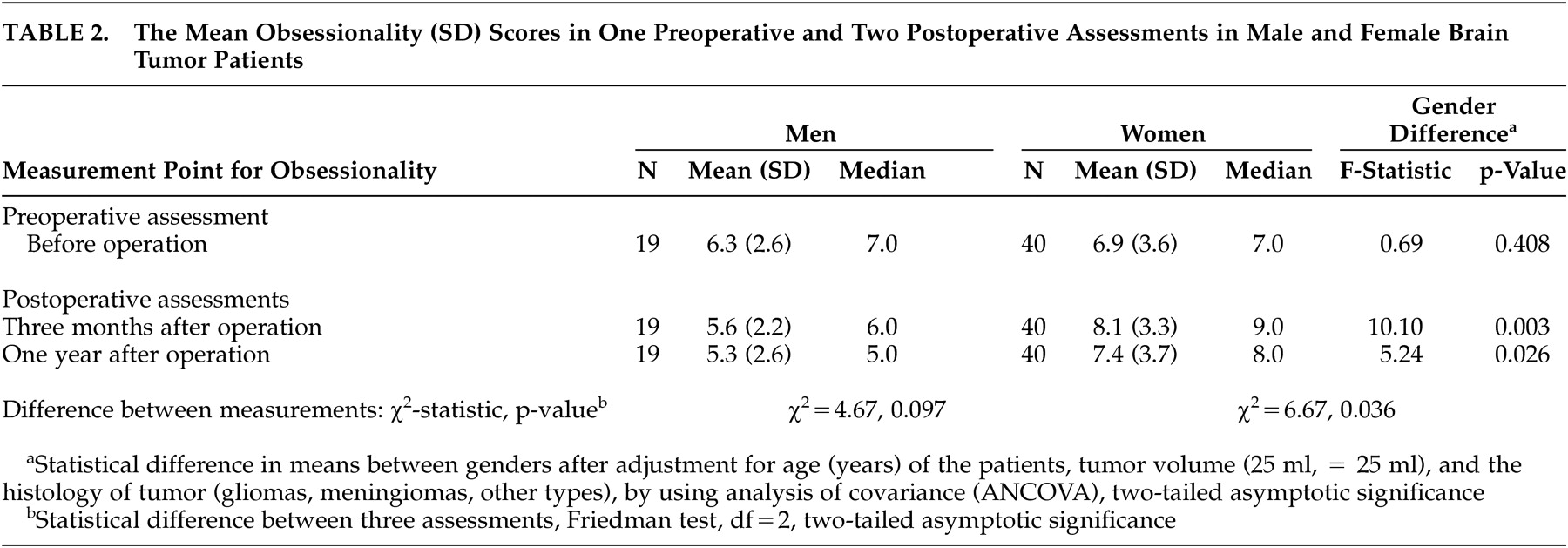
Table 2 shows the mean obsessionality scores and the results of gender comparisons in one preoperative and two postoperative assessments. Before tumor operation the mean obsessionality scores were 6.3 (SD 2.9) for men and 6.9 (SD 3.9) for women (no gender difference). Three months after surgical resection of the tumor, the mean obsessionality scores among women were statistically significantly higher 8.1 (SD 3.3) compared to the scores among men 5.6 (SD 2.2), (ANCOVA, p=0.003). A corresponding difference was seen also at 1 year after operation (ANCOVA, p=0.026).
When analyzing the change in individual score between three assessments of obsessionality, the increase in scores was statistically significant in women (Friedman Test, p=0.036). An opposite finding—a slight decrease in obsessionality scores between assessments—was seen in male patients, although the change indicated only a trend towards significance (Friedman test, p=0.097).
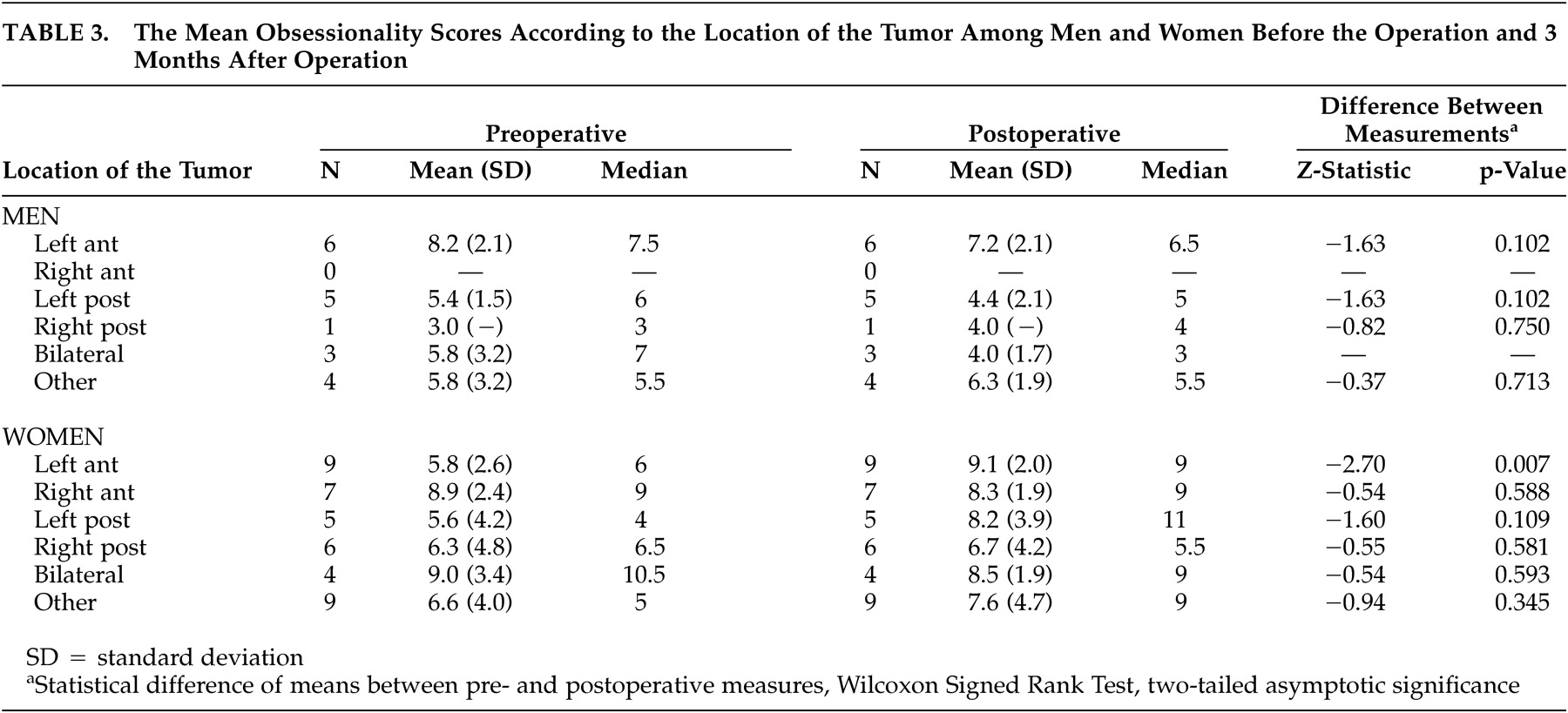
Table 3 shows the mean obsessionality scores according to the location of the tumor in preoperative assessments and 3 months postoperatively among male and female patients. The results of comparisons between preoperative assessments and those made 1 year postoperatively are not reported, since no statistically significant changes were observed between assessments made 3 months and 1 year after the operation in any of the comparisons according to gender and the location of the tumor.
In the female patients with a primary brain tumor in the left anterior hemisphere, a statistically significant increase in the level of obsessionality was observed between pre- and postoperative measurements (Wilcoxon signed rank test, p=0.007). No significant change in the level of obsessionality was seen if the tumor was located in any region other than the left anterior region in women and in any location in male patients.
DISCUSSION
Based on neurosurgical patients with a primary brain tumor, our main finding was that the mean obsessionality scores in women increased statistically significantly between measurements before operation and 3 months after operation compared to men. The differences remained significant until 1 year of follow up. The increased obsessionality scores in women reached scores indicating neurotic psychopathology of OCD.
According to Crown
15 and Crisp et al
20 there were no gender differences in the values of obsessionality in different study populations. Also, according to epidemiological studies the prevalence of OCD is roughly equal in men and women.
1 On the contrary, Zohar et al (1999) reported on sexual dimorphism of OCD.
22 In their opinion there are putative data showing that OCD is differently expressed in the two sexes and that the etiology of OCD might be different in the two genders. Thus, our finding of a statistically significant difference in the level of obsessionality between the genders is a novel and important finding and further studies on these topics are needed.
Another main finding in our study was that the increase in the level of obsessionality was statistically significant in women if the tumor was located in left anterior hemisphere. Previous case reports of male and female patients have stated that obsessions or compulsions were linked with lesions caused by brain tumor in frontal regions of the brain.
7,9 Our results support these case reports in relation to obsessionality and neuroanatomic brain location of the tumors. This is the first time so far that these preliminary findings published in case reports have been confirmed in a representative clinical patient sample.
Ward (1988) suggested that a focal cerebral disturbance could cause OCD that had earlier been considered to be purely psychological in etiology.
7 Nowadays OCD is considered to be a neuropsychiatric disorder in which altered function in the prefrontal-basal ganglia-thalamic-prefrontal circuits is particularly important.
23 Functional imaging has shown that OCD is characterized by increased activity in the orbitofrontal cortex and in basal ganglia at rest and especially during exposure to feared stimuli.
24 Furthermore, Tot et al (2002) found that patients with OCD had left frontotemporal dysfunction recorded by quantitative electroencephalography (QEEG), especially in women.
25In this study we were able to study subjects who simultaneously had brain dysfunction caused by a tumor, underwent surgery, and at the same time were probably frightened and worried. The pathophysiology of OCD has been linked with serotonin metabolism
26 and with metabolism of stress neuropeptides such as arginine vasopressin, corticotrophin releasing hormone, somatostatin and oxytocin.
27 However, there are a few studies on transmitters or neuropeptides related to brain tumors. Some studies have linked high levels of somatostatin receptors with gliomas and meningiomas.
28,29 In our study no information was available about biochemistry, i.e. the levels of stress hormones, serotonin or somatostatin in the patients. Thus, in further studies of the psychiatric disorders among brain tumor patients it is important to define the content of these transmitters and neuropeptides and to determine the density of these receptors on tumors.
The limitations of our study were that we could not evaluate the previous psychiatric history or premorbid personality of the patients. The fact that the number of cases in every subgroup was rather small was also a limitation of our study, although our database is a representative and unselected sample of the population in Northern Finland. Unfortunately the study protocol did not include an assessment of the exact psychiatric diagnosis according to any structured criteria-based diagnostic interview. Our tool of CCEI is a rough scale for measuring OCD and thus our findings are only suggestive in nature. New research is needed in which the diagnosis of OCD is confirmed by a structurized and standardized interview by a psychiatrist.
In summary, it is to our knowledge that this is the first study in which the occurrence of obsessionality among patients with a primary brain tumor has been studied in a representative sample of the population of a geographical area. We suggest that among patients with a primary brain tumor the level of obsessionality is associated with the removal of tumor in women, and especially if the tumor is located anteriorly in the brain. We emphasize also the important clinical aspect of this study: it is important to evaluate separately possible OCD symptoms because the patients are usually aware that their thoughts and behavior are strange, feel ashamed and try to hide their obsessions and compulsions.
26 Since increased obsessionality was found immediately, as early as 3 months after surgery, these psychiatric symptoms ought to be already evaluated in neurosurgical units. It is also known that if OCD can be prevented it has a positive effect on the patients’ quality of life.