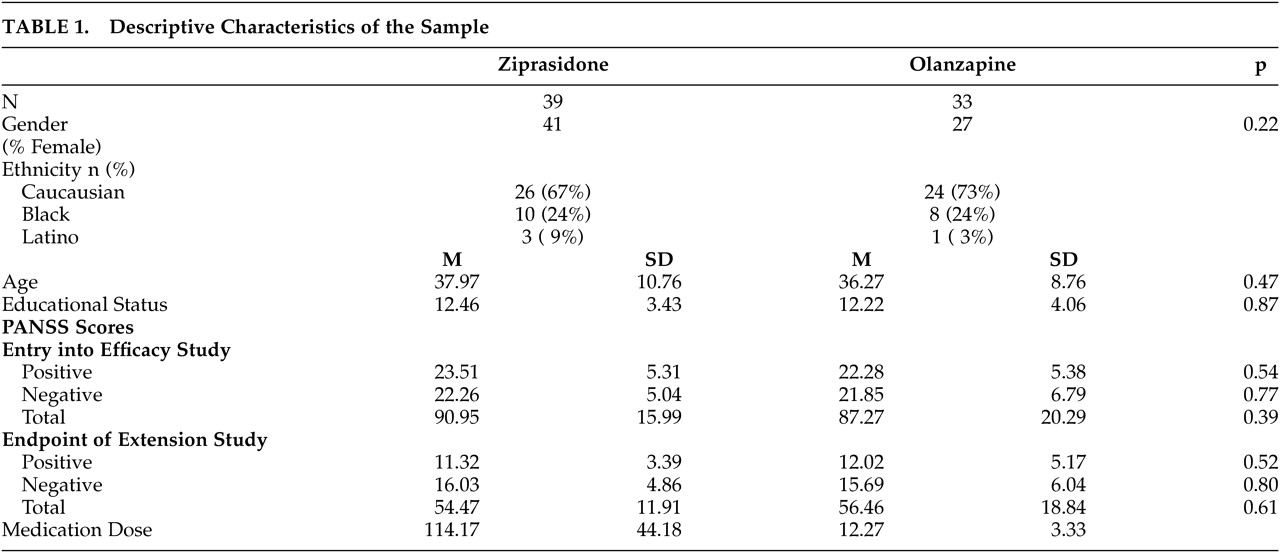
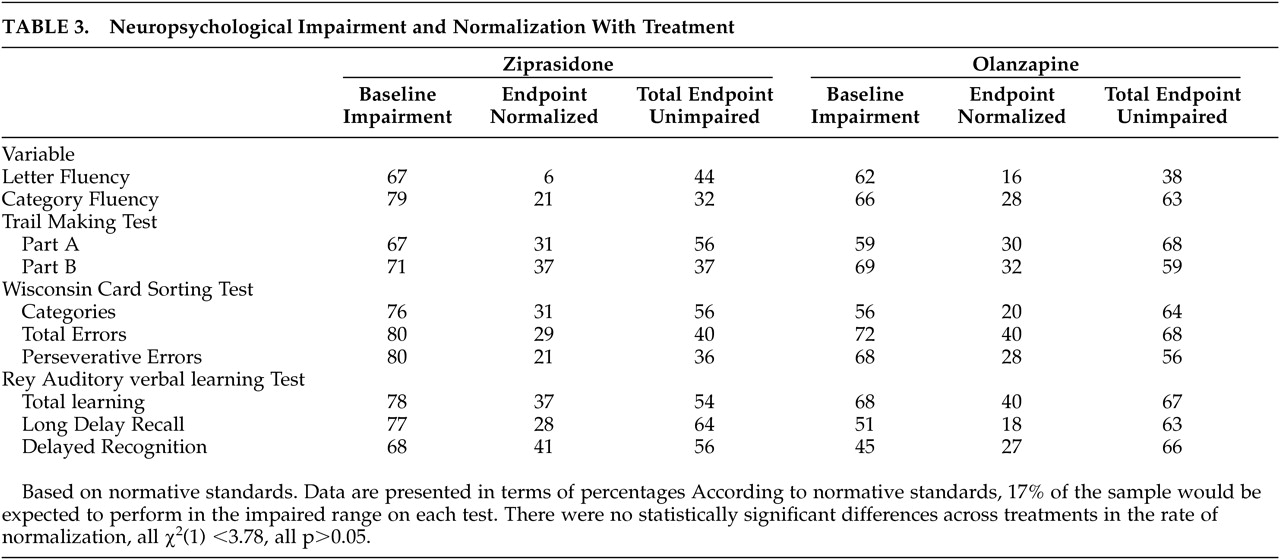
The results of this 6-month double-blind randomized extension trial are consistent with previous reports of the long-term effects of atypical antipsychotic treatment, although this study has several methodological advantages relative to those studies. Consistent with previous studies of the acute effects of olanzapine, risperidone, quetiapine, and clozapine, long-term treatment is associated with substantial cognitive benefits. The size of these changes is relatively large compared to short-term treatment studies. For example, for several of the variables examined, the level of improvement in performance for the patient group during the 6-month study was close to double that seen in typical short-term studies. The magnitude of short-term change in the previously published 6-week study was quite consistent with that reported in other large-scale double-blind studies comparing atypical antipsychotic medications.
5–6 While some of the changes in the present study seem quite large, it is important to note that the aggregate level of change across measures is quite consistent with the change scores reported by Velligan et al. (d=1.0)
9 in their open study of 6 months of treatment with quetiapine using a smaller assessment battery. In fact, if verbal fluency scores were not considered, the aggregate level of change would be over SD = 1.0 for both treatment groups. Also consistent with many previous studies
5–6,10–11 there was little correlation between clinical improvement and cognitive changes, suggesting independent dimensions of change. When comparing the size of the cognitive change effects in the current study to previous research, it is important to consider the similarity of the cognitive assessments. Most of the previous double-blind studies finding smaller effects of treatment used essentially identical assessments of processing speed (the Trail Making Test), verbal fluency, verbal learning, and executive functioning (see Keefe et al.
1 and Harvey and Keefe
2 for estimates of the effect sizes for previous changes) Thus, the greater effects in this study are not due to differences in instrumentation. For the majority of the cognitive variables for both treatment groups, it was found that the increases in the proportion of patients performing in the normal range at the end of the study were statistically significant. Further, using conservative criteria for normalization of performance, as many as 41% of the patients (depending on the measure) manifested a change in their performance that took them from outside the normal range of functioning to inside the normal range. When global cognitive functioning was examined, the overall change with treatment was quite substantial, especially when considering that verbal fluency performance was not markedly affected by either treatment. The number needed to treat (NNT) statistic for changing from impaired to unimpaired functioning across a variety of different cognitive measures ranges from a high of 18 (normalization of letter fluency with ziprasidone treatment) to a low of 2.5 (normalization of delayed recall with ziprasidone treatment). The average NNT across the different domains of cognitive impairment and the two treatments is approximately 4. This is a very low NNT by the typical standards of evidence-based medicine and compares very favorably with the NNT for treatment with clozapine to prevent a significant suicide attempt in schizophrenia (NNT=13)
26 and is also considerably better than the benefits of aspirin treatment to reduce cardiac events in individuals with a history of MI. Although these data indicate that olanzapine and ziprasidone have relatively similar cognitive effects, this finding does not mean that these drugs would be expected to have equivalent benefits for all patients. For instance, patients switched from risperidone, olanzapine, or conventional antipsychotic medications because of lack of efficacy or side effects experienced improvement in several cognitive domains.
27 Patients switched from olanzapine to ziprasidone in that study experienced improvements in variables associated with metabolic syndrome as well.
28 These data suggest that medications that appear equivalent on a group-mean basis may not be the optimal treatment for every patient with schizophrenia and that changing medications may lead to changes in clinical, cognition, and safety variables. There are some limitations of this design that require mention. First, only patients who experienced a clinical benefit from atypical antipsychotic treatment were entered into the extension, clearly influencing the sample of patients who were candidates for the study. This procedure may not be markedly different from clinical treatment algorithms, however, in that patients who fail to respond with clinically apparent reduction in their symptoms to 6 weeks of atypical antipsychotic treatment are often considered for a change in medications. An additional 20% of the patients did not provide endpoint cognitive data. Second, the sample size for the extension study is relatively smaller than that of the earlier acute treatment study. While this reduces the likelihood of finding significant between-groups treatment effects, the sample size was adequate to identify statistically significant changes in cognitive test performance from baseline across the two different treatment periods. Third, because there have been few successes in improving cognition in patients with schizophrenia, there are no consensus criteria for normalization of functioning. Our criteria may not be the only way to define neuropsychological normalization. Finally, it is possible that these changes are at least partially due to retesting effects. This is an important issue in many clinical trials, because use of placebo conditions is problematic and the result is often a parallel study comparing two approved treatments. This issue does require careful consideration. The results of Hawkins and Wexler
22 found that practice effects on the CVLT were twice as large from the baseline to first reassessment as they were from the first to second reassessments, with those changes not significant on a two-tailed basis. Further, they found no practice effects on the Trail Making Test. Also, the patients in the Hawkins and Wexler study were involved in concurrent cognitive remediation programs and performed much better on the memory assessment at baseline than the patients in this study. Second, recent data have suggested that patients with schizophrenia have more modest practice effects than those expected in the healthy population. In fact, the authors
29 have recently shown that older patients with schizophrenia treated with conventional antipsychotic treatments did not improve to a statistically significant extent on any test in a 21-test cognitive evaluation. Focusing on memory performance, the change from the baseline assessment to retest was not statistically significant, and an effect size of d=0.08 was reported, which is very small compared to the effect size reported in the Hawkins and Wexler study. Finally, a systematic study of practice effects on attentional performance indicated that patients with schizophrenia treated with low doses of conventional medications failed to manifest any appreciable improvement in performance with over 8000 practice trials.
30 That said, increasing the ability of patients with schizophrenia to improve substantially in cognitive functioning with practice and exposure is a goal of many interventions for patients with schizophrenia.
31 For instance, the goal of many cognitive remediation efforts is improvement in cognitive functioning, often as the results of extensive practice on cognitive measures.
32 Meta-analyses of the results of these interventions have often suggested that these practice effects are not substantial.
33 However, a recent study
34 has indicated that extensive cognitive training may induce normalization of memory functioning for some proportion of patients with schizophrenia, although there were no data regarding pharmacological status the patients presented in that paper. Thus, pharmacological interventions that facilitate behavioral or cognitive practice-related learning are a desirable treatment goal. This study does not demonstrate that these treatment-related changes are associated with improvements in functional outcome. In fact, the Velligan et al. study found that quality of life indices improved more substantially with long-term atypical treatments than scores on a functional status rating scale. It should be noted that the rating scale used by Velligan et al. the Heinrichs-Carpenter Quality of Life Scale (QLS),
35 has elements of both subjective QoL as well as objective indices of functional skills performance such as frequency of interpersonal interaction. At the same time, in order for these improvements in cognition to have functional significance, it must be demonstrated that the changes are related to objective indicators of improved community adjustment.