A number of reports suggest that among healthy elderly persons, nearly one-third complain of memory problems.
1 –
6 Clinicians often are faced with the choice of ignoring a subjective memory complaint and reassuring patients (which might lead to missing cases of early dementia) or taking such a complaint seriously (which could trigger unnecessary dementia evaluations). This dilemma may be particularly problematic with family members of patients with Alzheimer’s disease. Many biological relatives of Alzheimer’s disease patients recognize that they are at higher risk for Alzheimer’s disease,
7,
8 and even spouses of Alzheimer’s disease patients can become sensitized to the symptoms of memory impairments. Any information that helps clinicians better evaluate the likely etiology of such a complaint would be welcome.
Previous literature on subjective memory complaint has focused upon either healthy volunteers or population-based cohorts. To our knowledge, subjective memory complaint in the relatives of patients with Alzheimer’s disease has not been previously examined. Because cognitively healthy relatives of patients with Alzheimer’s disease are at higher risk for developing Alzheimer’s disease compared with non-relatives,
7 they provide a unique opportunity to further understand this phenomenon. The goal of this study was to identify the factors associated with subjective memory complaint among cognitively healthy first-degree relatives of Alzheimer’s disease patients.
DISCUSSION
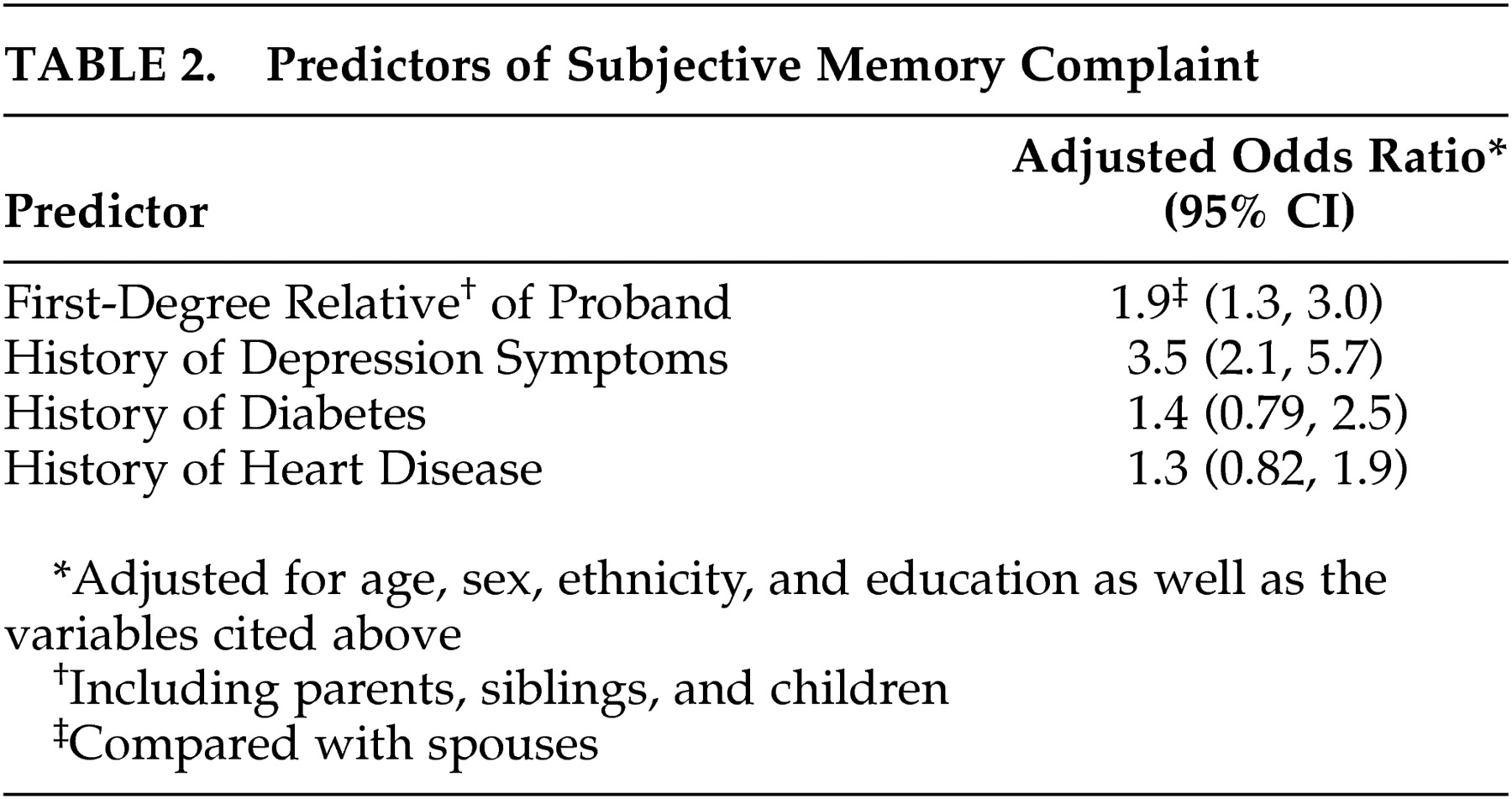
Both first-degree biological kinship and depression symptoms were predictors of subjective memory complaint in cognitively healthy family members of patients with Alzheimer’s disease. First-degree relatives were nearly twice as likely to report subjective memory complaint, and relatives reporting a history of depression symptoms were more than three times as likely to report subjective memory complaint. Subjective memory complaint has been associated with increased risk of subsequent dementia in some, but not all studies. In a large sample of healthy elderly Dutch, subjective memory complaint was significantly associated with the development of dementia within 3 years.
17 This association was stronger in those subjects who had both memory complaints and poor memory performance. In an Australian community sample, subjective memory complaint did not appear to predict cognitive change or the development of dementia over nearly 4 years of follow-up, once anxiety and depression symptoms were taken into account.
18 However, we found the opposite results when we reanalyzed their data with more sophisticated modeling techniques.
19 A recent longitudinal study used a single question to assess memory complaints with an average follow-up of 3.2 years and found that subjective memory complaint was a relatively strong predictor of incident Alzheimer’s disease in older persons in whom cognitive impairment was not yet apparent.
20 In a review of clinical and population-based studies, Jonker et al.
21 reported an association between memory complaints and memory impairment in elderly subjects, after adjustment for depressive symptomatology.
In several community-based studies, subjective memory complaint has been found to be associated with depression symptoms,
22,
23 and in the MIRAGE data set (the same data set utilized for this study), depression symptoms occurring even 25 years prior to dementia were associated with an increased risk of developing Alzheimer’s disease.
15 These reports suggest that depression symptoms cannot only be part of the preclinical phase of Alzheimer’s disease, but may also be an early risk factor for later development of Alzheimer’s disease.
Our participants were only evaluated at one point in time; thus, we cannot comment on the predictive value of subjective memory complaint. However, we have shown two significant associations with subjective memory complaint among relatives of patients with Alzheimer’s disease. Consistent with some of the cross-sectional studies above, subjective memory complaint among cognitively healthy relatives of Alzheimer’s disease patients was associated with a prior history of depression symptoms. Since memory complaint (with or without memory impairment) is a common symptom of depression,
24,
25 this suggests that subjective memory complaint in the presence of healthy cognition should trigger an evaluation for depression.
Subjective memory complaint was also more common among first-degree biological relatives of Alzheimer’s disease patients than among their spouses. The interpretation of this is unclear. One explanation is that since biological relatives of Alzheimer’s disease patients are at higher risk of developing Alzheimer’s disease than are spouses of Alzheimer’s disease patients,
7 the higher proportion of subjective memory complaint could reflect the sub-clinical manifestation of Alzheimer’s disease. Alternatively, since Alzheimer’s disease is now widely recognized as having a heritable component,
7,
26 biological relatives may be more sensitized to memory lapses than spouses and may report them more frequently.
Our study is limited by the cross-sectional nature of the data collection, as noted above. Cognitive testing to confirm self-report of normal cognition was only available in a subset of the participants; however, the correlation between self-report and telephone cognitive testing was very high. The use of a single-item, self-reported measure to examine a complex construct, such as depression, may not be accurate enough to avoid misclassifications. Moreover, we have emphasized that we assessed “depression symptoms” rather than depression. Nonetheless, previous research on depression screening has found that a two-item measure,
27 or even a single question,
28 can be an effective screen for depression. In our study, relatively few participants endorsed subjective memory complaint compared with population prevalence studies that have reported a prevalence of 25% or more.
29 Self-selection may have resulted in a healthier sample with fewer complaints of memory problems than in the general population. However, to our knowledge this is the first study to examine the predictors of subjective memory complaints in cognitively healthy relatives of Alzheimer’s disease patients. Longitudinal studies of relatives are needed to define the association between subjective memory complaint and Alzheimer’s disease in this at-risk population.
Based on what is known about the predictors of subjective memory complaints among cognitively healthy relatives of patients with Alzheimer’s disease, when family members present such complaints, clinicians should evaluate them for memory impairment, since memory complaints may herald the onset of Alzheimer’s disease. If objective memory testing reveals no impairment, then depression should be considered as an alternative explanation for the complaint.