A debatable yet promising area in schizophrenia research involves the use of cognitive and sensory system dysfunction as endophenotypic measures of illness.
1 Cognitive deficits are reported in patients with schizophrenia and often in their healthy first-degree relatives. These abnormalities range from early stages of information processing to impairments in higher-level cognitive functions. Early sensory dysfunction includes abnormal physiological responses in tasks such as pre-pulse inhibition, smooth-pursuit eye movement, and antisaccade eye movement.
2 –
5 Diminished performance in working memory, verbal memory, word fluency, bilateral motor skills, executive functions, sustained attention, and affect recognition reflect abnormalities at higher cognitive processing levels.
6 –
12 As sensory and cognitive deficits are evident in patients and their healthy first-degree family members, they may provide valid and specific vulnerability markers of schizophrenia.
Olfactory function is one potential vulnerability marker currently being investigated. Several studies have examined abnormalities in odor identification and odor detection threshold sensitivity in patients with schizophrenia.
13 –
15 Unlike their cognitive neuropsychological deficits, which are relatively static over the course of illness, patients’ olfactory abilities appear to decline linearly over time, independent of the effects of aging.
16,
17 Similarly, family and twin studies document odor identification deficits in healthy monozygotic twins discordant for schizophrenia and in first- and second-degree relatives of patients with schizophrenia.
11,
18,
19Neuroanatomical deficits reported by Turetsky et al.
20,
21 suggest that both patients and their healthy first-degree family members show decreased volume in the olfactory bulb and primary olfactory cortex relative to healthy volunteers. Notably, patients show decrements in both olfactory bulbs as well as in the left and right perirhinal and entorhinal cortices, while the reduction in relatives is lateralized to the right olfactory bulb and specific to the entorhinal cortex only; however, family members also exhibit an increase in the left perirhinal cortex compared to patients.
22 Furthermore, more recent studies by Rupp et al.
23 report bilateral reductions in hippocampus and amygdala volumes and associate smaller hippocampal volumes with poorer odor discrimination ability.
Overall, these previous reports suggest that, in schizophrenia, both pathological neurodegenerative and genetically mediated neurodevelopmental processes affect olfactory processing and the brain regions subserving olfaction. The study of olfaction, therefore, may advance our understanding of the neurodevelopmental origins of schizophrenia, the factors contributing to genetic vulnerability, and the pathophysiology of disease progression.
Though previous psychophysical studies of schizophrenia patients and their family members have demonstrated a significant genetic contribution to the odor identification deficit, the interpretation of these findings has been complicated by diagnostic heterogeneity in the patient samples studied and by the inclusion of some first- and/or second-degree family members who had Axis I and Axis II disorders.
11 Furthermore, a majority of studies to date have examined olfactory abilities via birhinal assessment. Considerable bilateral interaction in the olfactory system has been shown,
24 and the isolation of the contribution of each nostril (and hemisphere) via unirhinal presentation may help provide further information concerning the nature of the chemosensory deficit observed in schizophrenia.
An example of bilateral interaction can be seen in a study of odor recognition memory in healthy comparison subjects,
25 where memory scores were better during birhinal odor presentation than during unirhinal testing. These findings provide the impetus for examining unirhinal olfactory performance in schizophrenia patients and in their healthy first-degree relatives.
In summary, though it has been consistently reported that olfactory brain structures and psychophysical olfactory performance are abnormal in patients with schizophrenia, the findings in healthy first-degree family members remain unclear. Therefore, our study assessed odor identification and odor detection threshold sensitivity in patients with a sole diagnosis of schizophrenia and in their healthy first-degree family members. Based on previous evidence, we expected to find a reduction in olfactory performance in patients and first-degree family members in both nostrils, with greater deficits lateralized to the right nostril in family members. All olfactory stimuli were presented unirhinally to capitalize on the unique ipsilateral projections of this sensory system. Such an approach would isolate each nostril and hemisphere and may prove to be more precise than birhinal presentation and may reveal further insight about the genetic vulnerability of schizophrenia.
METHOD
Participants
Participants comprised 22 patients with a DSM-IV
26 diagnosis of schizophrenia (10 men, 12 women), 30 healthy first-degree family members (16 men, 14 women) and 45 healthy comparison subjects (21 men, 24 women). A total of 22 distinct family groups were included in the study. This included six parents, 23 siblings, and one offspring of patients with schizophrenia. A subset of these subjects (11 patients, 19 family members, seven healthy comparison subjects) were included in our previous study of olfactory bulb volume decrements in first-degree family members.
27 All participants were recruited from the Schizophrenia Research Center (SRC) at the University of Pennsylvania Medical Center, Philadelphia. The three groups did not differ with regard to age (
F [2, 94]=1.20, p=0.30), sex (χ
2 =0.42, df=2, p=0.81), or level of education achieved (
F [2, 94]=1.72, p=0.19). We collected the current smoking status of the three groups and evaluated smoking by computing a “pack-years” score, based upon the number of cigarettes smoked per day and the number of years a particular individual had been a smoker. Since pack-years of smoking were not normally distributed, we used the nonparametric Kruskal-Wallis test to compare pack-years among the three groups. No significant difference was found (χ
2 =2.83, df=2, p=0.24). Furthermore, subjects were not allowed to smoke for at least 30 minutes prior to olfactory testing.
All subjects received a psychiatric interview (Structured Clinical Interview for DSM-IV, Patient or Nonpatient edition)
28,
29 and physical examination, including routine laboratory tests. We rated patients on the Brief Psychiatric Rating Scale (BPRS),
30 the Scale for Assessment of Negative Symptoms (SANS),
31 and the Scale for Assessment of Positive Symptoms (SAPS).
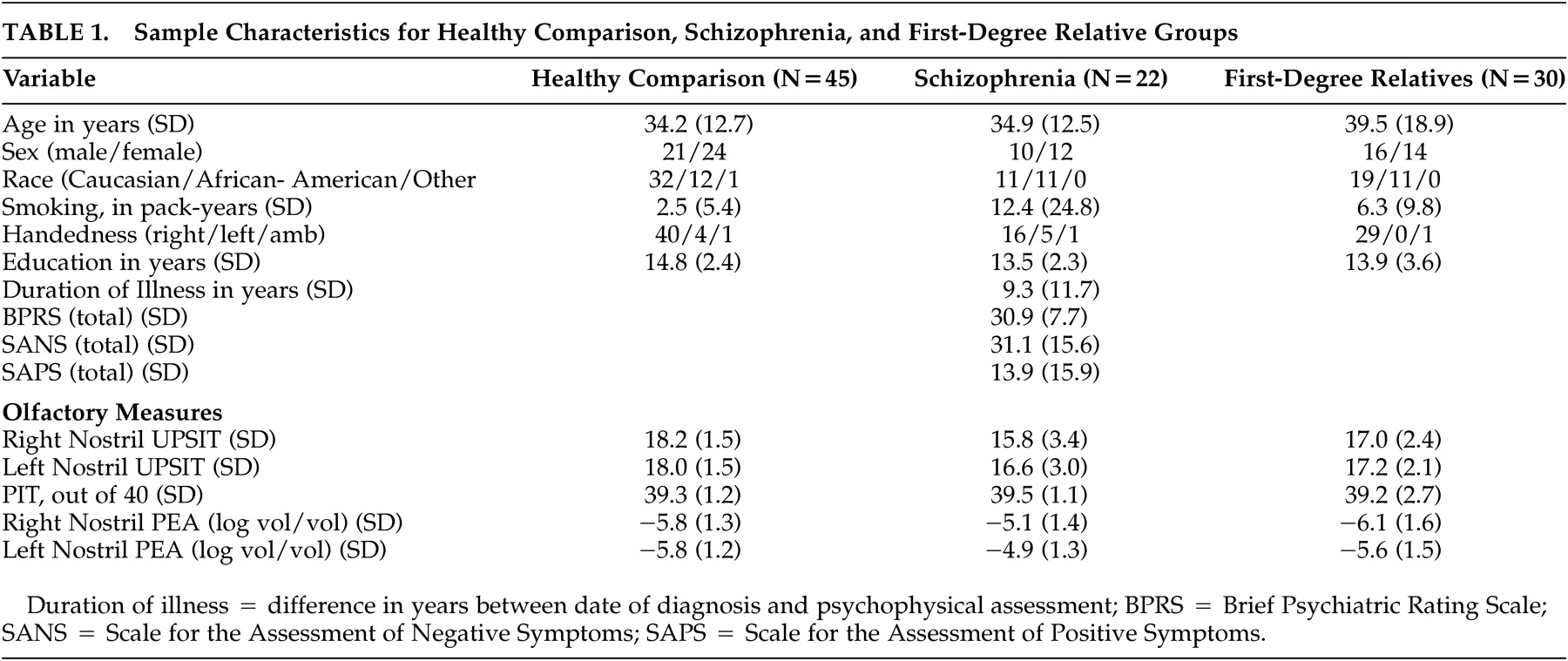
32 Ratings were completed by investigators trained to a criterion reliability of 0.90 (intraclass correlation). Twenty-one patients received SANS assessment, 20 patients received SAPS testing, and 20 received BPRS ratings. Demographic and patient clinical data are presented in
Table 1 .
Nine patients received atypical medications, nine received typical medications, one patient received a combination of atypical and typical medications, two patients were not medicated, and one patient had no record of current medications at the time of olfactory testing. The patients were all stable outpatients at the time of testing.
Family members were assessed for Axis II psychopathology with the Structured Clinical Interview for Personality Disorders.
33 In order to be included as a healthy first-degree family member, the individual had to meet specific criteria: 1) be the father, mother, offspring, or full sibling of an individual affected with a sole diagnosis of schizophrenia; 2) if a sibling, the individual must have shared the same mother and father as the individual affected with schizophrenia; 3) be currently and historically free of any Axis I or II psychiatric disorder; 4) meet all other inclusion criteria for participation in olfactory assessment. In addition to the Structured Clinical Interview for DSM-IV (SCID), healthy comparison subjects also received a structured interview for the assessment of Axis II psychopathology (SCID-II).
33 Based on these assessments, healthy comparison subjects were free of any Axis I or Axis II disorder and were negative for any family history of psychiatric illness.
Exclusion criteria for all subjects included: a history of neurological disorder, including head trauma with loss of consciousness; a history of substance abuse or dependence (as assessed by history, record review, and urine toxicology); any medical condition that might alter cerebral function; a recent respiratory infection or any other condition that could affect olfactory performance (e.g., common cold, allergies, significant septal deviation). Written informed consent was obtained after the procedures had been fully explained. This research complied with the Declaration of Helsinki, Finland’s ethical standards in the treatment of human research participants.
Assessment of Olfactory Function
All tests of olfaction were administered unirhinally (i.e., each nostril separately). Prior to presentation of the test stimuli, the nostril not being assessed was occluded with Durapore
TM tape (3M Corporation, Minneapolis, MN) fitted tightly over the edge of the nostrils and columna. This procedure effectively isolates the nostril under examination and prevents retronasal airflow.
25Odor Identification
Odor identification performance was assessed using the University of Pennsylvania Smell Identification Test (UPSIT).
34,
35 The UPSIT is a standardized, four-alternative, forced-choice test of odor identification comprising of four booklets containing 10 odorants apiece, one odorant per page. The stimuli are embedded in “scratch and sniff” microcapsules that are fixed and positioned on strips at the bottom of each page. A multiple-choice question with four response alternatives for each item is located above each odorant strip. The UPSIT was administered individually by a trained technician, who released the microencapsulated stimuli, placed them under each participant’s nostril, and recorded the answer following the participant’s response. Two booklets of 20 items were presented to the right nostril and the remaining two booklets were presented to the left nostril; the order of booklet and nostril presentation was systematically counterbalanced to avoid position effects.
Following administration of the test, subjects were given the Picture Identification Test (PIT).
36 The PIT is identical to the UPSIT in item composition and response characteristics, except that line drawings instead of odors are presented. This test was designed to screen for individuals with cognitive deficits that may confound UPSIT scores.
Odor Detection Threshold Sensitivity
All subjects received a single staircase, forced-choice odor detection threshold test to estimate basal detection sensitivity to phenyl ethyl alcohol (PEA: Gold Label Grade, Aldrich Chemical Co., Milwaukee, WI), a compound with low trigeminal stimulation properties
34,
37 in each nostril. In this procedure, the staircase began at the −6.00 log concentration step of a half-log step (vol/vol) dilution series, extending from the weakest −10.00 log concentration to the strongest −2.00 log concentration. Initially, it was moved upward in full-log steps until correct detection occurred on five sets of consecutive trials at a given concentration level. If during this initial phase, an incorrect response was given on any trial, the staircase was moved upward a full-log step. Once the criterion of five correct consecutive responses was made, the staircase was reversed and subsequently moved up or down in half-log increments, depending upon the subject’s performance on two pairs of trials (i.e., each pair consisting of a choice between diluent and odorant) at each concentration step. The geometric mean of the last four staircase reversal points of a total of seven served as the estimate of threshold sensitivity.
27Statistical Analysis
To test for differences in UPSIT and PEA performance, we performed linear regression analyses including between-group effects of age, sex, and psychiatric diagnosis, and a within-group effect of nostril, plus interactions of diagnosis by nostril and diagnosis by sex. By definition, regression analysis requires independence of samples. In this case, the patient and family member groups are not truly independent; thus, a standard linear regression would tend to underestimate the true variances and, in turn, would provide falsely low p-values. To accommodate properly for this feature of the data in a regression framework, we used generalized estimating equations (GEE) for regression.
38 The GEE regression model, which is analogous to a repeated measures analysis of variance (ANOVA), has one dependent variable, with the repeated measures accounted for by dummy-coded variables. The GEE regression model is ideal for the proposed analyses as this technique can accommodate for nonindependence among groups in this repeated measures data.
The GEE methodology adjusted for clustering by family members, using the compound symmetry correlation structure which assumes that any two distinct observations from the same family have the same correlation coefficient. The interaction terms tested whether the relationship between diagnosis and olfactory performance differed by sex or by nostril. When the interactions were not statistically significant, the models were rerun, including only the main effects. Similar models have been employed in analyses of olfactory measures in monozygotic twins discordant for schizophrenia.
18 Although the effects of smoking on olfactory psychophysical measures have been shown to be negligible in patients with schizophrenia,
13,
14 analyses including smoking status (pack-year) as a covariate did not reveal any significant influence on the reported results. Bonferroni adjustments were made to p-values to adjust for multiple comparisons.
RESULTS
Results of the GEE analysis revealed a significant difference in odor identification performance among the three groups (χ
2 =11.07, df=2, p=0.004), with no interactions of sex (p=0.59) or nostril (p=0.28) being observed. Post-hoc comparisons indicated that healthy comparison subjects performed better on the UPSIT than both patients (χ
2 =9.08, df=1, p=0.003, Bonferroni p=0.008) and healthy family members (χ
2 =5.68, df=1, p=0.017, Bonferroni p=0.05). Notably, patients and family members did not differ significantly from each other on UPSIT performance (χ
2 =2.51, df=1, p=0.11, Bonferroni p=0.34) (Upper panel,
Figure 1) .
Analysis of PEA thresholds also showed significant differences among the three groups (χ
2 =6.76, df=2, p=0.034). Pair-wise comparisons revealed that patients had significantly poorer PEA thresholds relative to healthy comparison subjects (χ
2 = 6.71, df=1, p=0.01, Bonferroni p=0.029). In contrast, there were no significant differences in the ability to detect PEA between healthy participants and family members (p=0.84), or between patients and family members (p=0.06) (Lower panel,
Figure 1 ).
We observed no significant correlations between olfactory measures and clinical rating scales (SANS, SAPS, BPRS) in patients. The three groups did not differ with regard to PIT performance ( F (2, 59)=0.13, p=0.88).
DISCUSSION
To the best of our knowledge, the results of the present study provide the first description of unirhinal abnormalities of olfactory function in a relatively large sample of patients with schizophrenia and their healthy first-degree relatives. This study expands upon previous birhinal findings that characterize olfactory abnormalities in patients and biological relatives. Specifically, both patients and first-degree family members showed significant deficits in their ability to identify odorants correctly on the UPSIT relative to healthy comparison subjects. Consistent with other studies of unirhinal odor identification in schizophrenia, we observed no interaction with nostril and diagnosis. These data are in agreement with recent findings of structural abnormalities in the olfactory bulb
21 and primary olfactory cortex
22 in healthy first-degree family members, and suggest that abnormalities in the olfactory system are represented both structurally and behaviorally in these subjects. A previous report of birhinal odor identification performance indicated that family members’ scores were statistically intermediate to those of patients and comparison subjects.
11 In our study, the mean scores of family members were nominally intermediate to those of patients and comparison subjects; however, family members’ UPSIT scores were not statistically different from the patients'. Thus, the finding of comparable odor identification deficits in patients and healthy first-degree relatives suggests a more significant genetic influence on olfactory processes. Hence, measurement of unirhinal odor identification performance may serve as a more efficacious method of establishing genetic vulnerability to schizophrenia than more commonly reported birhinal performance measures. Similar findings were reported in a study of birhinal odor identification in monozygotic twins discordant for schizophrenia,
19 in which affected and healthy twins differed from healthy comparison subjects on the UPSIT but did not differ from one another.
With regard to odor detection threshold sensitivity, patients showed a significant impairment in PEA detection thresholds for both nostrils, relative to healthy comparison subjects. This finding suggests that the deficit in odor identification is accompanied by similar impairment in basic detection thresholds. Indeed, the effect sizes for patient and healthy comparison group differences on the UPSIT (
d + =0.83, 95% CI=0.30–1.36) and those for PEA thresholds (
d + =0.70, 95% CI=0.18–1.23) were not significantly different from each other (Q
B (1)=0.12, p=0.73). These data are consistent with previous meta-analytic work indicating more diffuse impairment across different domains of olfactory function in patients with schizophrenia.
14 The reported differences could not be explained by smoking status or smoking duration, as inclusion of these factors in the GEE analysis did not alter the observed findings. The presence of both identification and threshold sensitivity deficits in patients, but only identification deficits in the healthy family members, may indicate a dissociation between olfactory deficits that represent genetically mediated vulnerability factors and deficits that are manifestations of the overt disease process. It must be noted in this regard, though, that the reliability of threshold detection sensitivity tests is somewhat lower than that of odor identification tests, and this may simply reflect the reduced power of threshold detection tests to detect lower level abnormalities in the context of greater test variability.
39The psychophysical data from a subset of these subjects were also included in our previous report of olfactory bulb volume decrements.
21 The results of that study differed, to some extent, from those reported here. In that study, patients’ UPSIT scores were not significantly reduced, relative to those of healthy comparison subjects, though their mean scores were lower. Also, they exhibited significant reductions in odor detection threshold sensitivity compared to their unaffected family members; in our study, this difference just failed to reach statistical significance (p=0.06). It should be emphasized that the sample sizes in our previous study were approximately half of those included here. Consequently, there was less power to detect statistically significant differences. The increased sample size in this study allowed for greater power and accuracy, and this likely is the best explanation for any observed differences, especially for the previous failure to find a patient deficit on the UPSIT.
A number of reasons may exist for the described differences from the Kopala et al.
11 study of family members. First, the current investigation examined unirhinal odor identification and odor detection threshold sensitivity performance, whereas Kopala examined birhinal abilities. As stated earlier, unirhinal testing relatively isolates each nostril and minimizes any potential bilateral facilitation. As bilateral interaction has been reported for the olfactory system,
24 a difference between birhinal and unirhinal presentation and performance would be expected. Second, the current study examined patients with a sole diagnosis of schizophrenia and their healthy, first-degree relatives. The Kopala et al. study sample comprised a mix of diagnoses in the psychotic family member group (i.e., schizophrenia, schizoaffective disorder, psychosis not otherwise specified), as well as major depressive episode, alcohol abuse, and Axis II disorders in some members of the nonpsychotic family sample. Third, the sample size in the current investigation was also somewhat larger than the Kopala study and, in conjunction with a more homogeneous first-degree family sample, may have had greater power to discern the reported psychophysical differences. Regardless, both studies support a significant genetic contribution to the odor identification deficit observed in patients with schizophrenia. Finally, lateralized impairments in odor identification and detection threshold sensitivity were not observed in the current data. This finding is consistent with other studies examining unirhinal olfactory performance which have not described significant diagnosis by nostril interactions.
40 –
43 Good et al.,
42,
43 however, using a categorical classification algorithm derived from normative data, did identify subgroups of patients who showed lateralized deficits in odor identification. The application of this strategy in larger samples may warrant consideration.
Genetic contribution to the etiology of schizophrenia has been demonstrated in epidemiological studies. The presence of a genetic vulnerability, however, need not result in clinical disease. The precise nature and mode of inheritance of this genetic liability are not clear, and individuals who are genotypically at risk for schizophrenia cannot be readily identified. One approach to this problem is to explore biological and behavioral markers that might denote an increased genetic predisposition to the disorder, even in the absence of overt symptomatology. The identification of such “latent traits” or “endophenotypic” markers can contribute to our understanding of the disorder by illuminating the mechanisms of gene action as distinct from the clinical phenotype. This, in turn, can facilitate linkage analyses designed to identify the associated gene locus or loci, as well as the possible development of prevention and rehabilitation strategies. The current data support the notion that unirhinal odor identification deficits aggregate in healthy first-degree family members and may serve as a strong endophenotypic vulnerability marker.
Acknowledgments
This work was supported by NIMH grants MH-63381 to Dr. Moberg, MH-59852 to Dr. Turetsky, MH-66121 and MH-42191 to Dr. Gur, M01-RR00040 to the University of Pennsylvania General Clinical Research Center (GCRC), and an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression to Dr. Moberg. Special thanks to Ms. Janice and Mrs. Constance Lieber for their support of this research.