T he effects of HIV infection on brain function range from subtle cognitive impairment to frank dementia, the latter referred to as the AIDS dementia complex.
1 The neuropsychological profile of patients with AIDS dementia complex (ADC) is generally consistent with a “frontal-subcortical” pattern of impairment. As such, affected cognitive domains include fine motor coordination and speed, sustained attention, processing speed, executive function, learning efficiency, and working memory.
2 –
10 By contrast, functional cortical impairment (e.g., aphasia, apraxia, anomia) is not characteristic of HIV-related brain dysfunction, at least among patients with relatively mild to moderate symptoms.
Consistent with the clinical expression of cognitive symptoms, pathological abnormalities associated with HIV are usually confined to the deep gray and white matter, but as discussed below, may also be seen in the cortex. The severity of clinical disease generally correlates most strongly with structural and functional abnormalities in the basal ganglia and white matter and is influenced by the expression of a variety of host and viral factors in these regions.
11 –
13 Structural and functional neuroimaging studies have shown that alterations in subcortical volume or metabolism, particularly in the basal ganglia, are strongly related to cognitive dysfunction.
14Despite the converging evidence in support of a subcortical profile associated with HIV, there is neuropathological evidence that cortical abnormalities also exist among infected patients.
15 –
19 Reductions in cortical synaptic density have been reported at different stages of HIV infection,
20 and cortical dendritic branching is reduced among individuals with mild HIV-associated cognitive motor disorder.
21 These findings question the specificity of subcortical abnormalities in HIV as they relate to cognitive function.
Studies show that infiltration of the brain by infected macrophages is associated with an inflammatory cascade mediated by the release of host- (e.g., cytokines, oxygen free radicals) and viral-related factors (e.g., tat, gp120) that disrupt neural function.
22,
23 This neurochemical cascade is associated with metabolic abnormalities that can be detected and directly measured in brain tissue by proton magnetic resonance spectroscopy (MRS). Proton MRS provides a robust method to examine such neurochemical changes at the regional and cellular level. Previous studies using MRS have focused on several specific brain metabolites including choline, myoinositol, and N-acetylaspartate. Choline and myoinositol are believed to represent markers of gliosis, whereas N-acetylaspartate is a putative marker of mature neurons.
24 Studies of HIV patients have revealed significant elevations in choline and myoinositol in subjects with mild cognitive impairment and significant reductions in N-acetylaspartate in the cortex of individuals with more severe neuropsychological impairment.
24 –
38We formed an HIV MRS consortium to further assess the in vivo effects of HIV infection on regional brain function. The consortium developed a multicenter protocol that can reliably generate metabolite data at a short echo time, allowing for measurement of the myoinositol peak from three brain regions, specifically, the basal ganglia, frontal white matter, and parietal cortex. Initial outcomes from the consortium revealed that ADC patients exhibited significantly lower levels of N-acetylaspartate/creatine in the frontal white matter compared with the nondemented HIV-positive individuals and significantly increased levels of choline/creatine and myoinositol/creatine in the basal ganglia and frontal white matter compared with HIV seronegative subjects.
38 By contrast, nondemented HIV-positive individuals showed a significantly elevated myoinositol/creatine in the frontal white matter compared to seronegative subjects, and this was the sole metabolite abnormality.
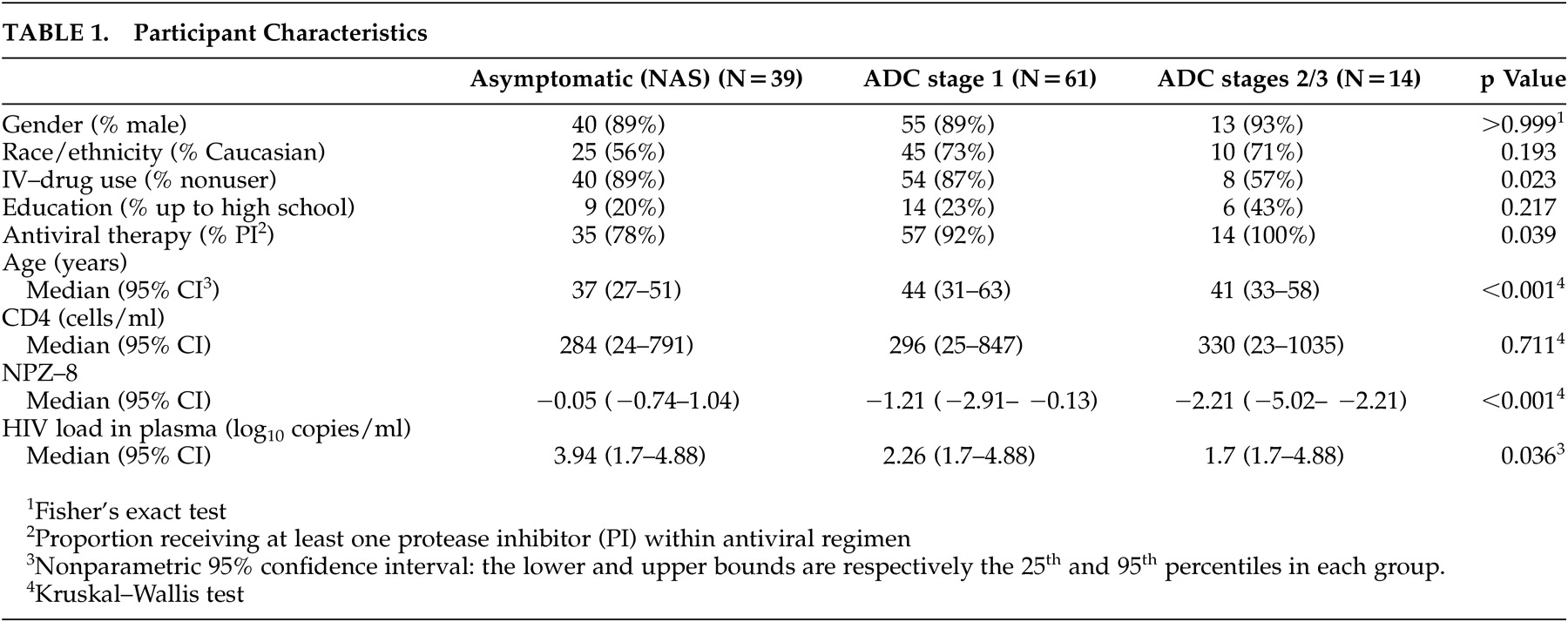
The purpose of the present study was to examine relationships between metabolite abnormalities in the basal ganglia and frontal white matter and neuropsychological performance in patients with HIV in the context of antiretroviral therapy. We examined the specificity of relationships between MRS indices obtained from the basal ganglia and frontal white matter and neuropsychological performance by determining whether neuropsychological performance correlated with MRS indices obtained in the cortex. The parietal cortex was selected as a nonspecific cortical site, since, based on previous studies,
38 this site is believed to be less involved in HIV and therefore would serve as a general index of cortical health. We predicted that neuropsychological function would correlate strongly with levels of specific metabolites in the basal ganglia, subcortical white matter, or both. In contrast, we hypothesized that neuropsychological function would not strongly correlate with metabolite levels in the parietal cortex.
DISCUSSION
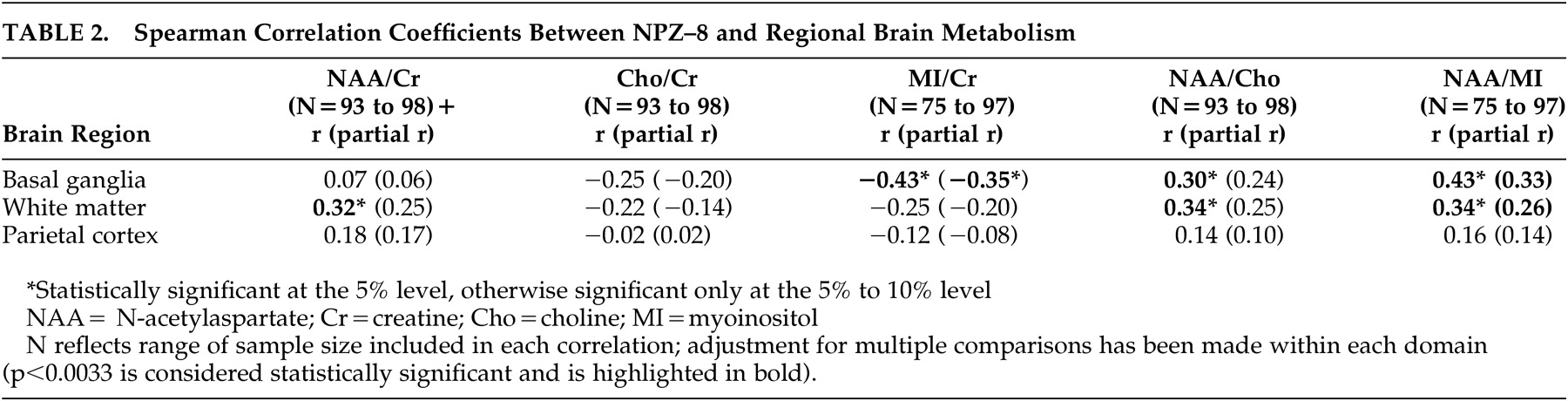
We undertook this study to examine the relationship between in vivo brain metabolism as measured by MRS and neuropsychological domains of cognitive function previously shown to be affected among HIV-infected patients. Strong relationships were found between neuropsychological performance and MRS indices of increased gliosis and reduced mature neurons in the subcortical regions, whereas no significant relationships were identified between these measures in the parietal cortex.
A number of previous studies have reported significant relationships between brain metabolite levels and cognitive performance in HIV. Rottenberg et al.
51 showed that decreases in glucose metabolism in the subcortical regions as measured by positron emission tomography correlated with measures of motor function, such as the Grooved Pegboard, while cortical metabolic abnormalities predicted performance on tests of executive function. López-Villegas et al.
33 applied high-resolution spectroscopy in the frontal gray and white matter in 15 HIV-positive subjects and found significant correlations between an aggregate MRS score based on an increase in myoinositol/creatine and a decrease in N-acetylaspartate/creatine and performance on Grooved Pegboard, finger tapping, Trail-Making Test, Part A, and Digit Symbol. Using a multivoxel imaging approach, Meyerhoff et al.
52 reported elevated choline in subcortical brain regions among cognitively intact individuals, and reduced N-acetylaspartate in subcortical brain regions among individuals with severe cognitive impairments. Specific relationships were observed between N-acetylaspartate levels in the subcortical brain regions and performances on measures of abstraction, immediate and delayed memory, verbal fluency, and fine motor dexterity. Finally, Chang et al.
53 reported significant relationships between elevated myoinositol to creatine levels in the frontal white matter and performance on executive tasks among HIV-positive individuals naïve to HAART.
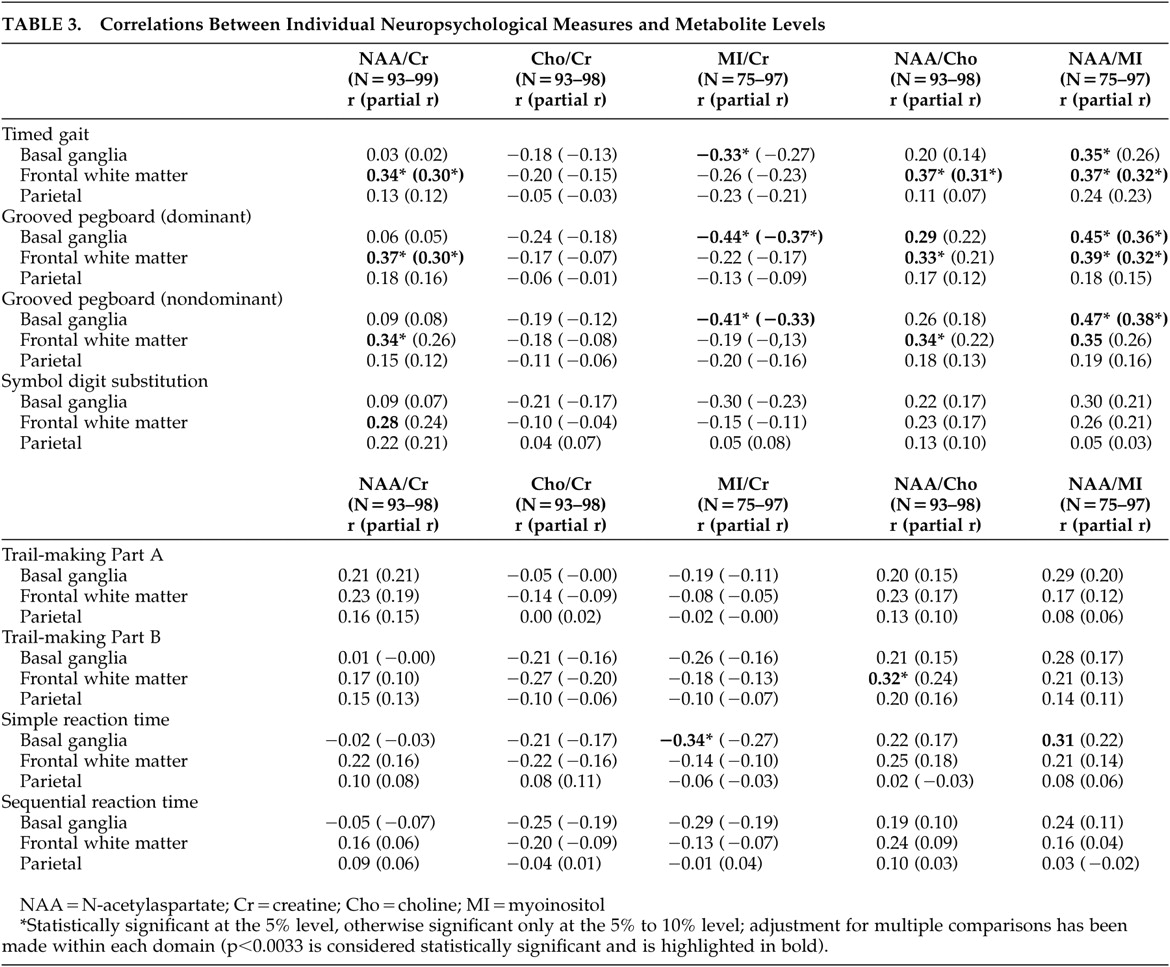
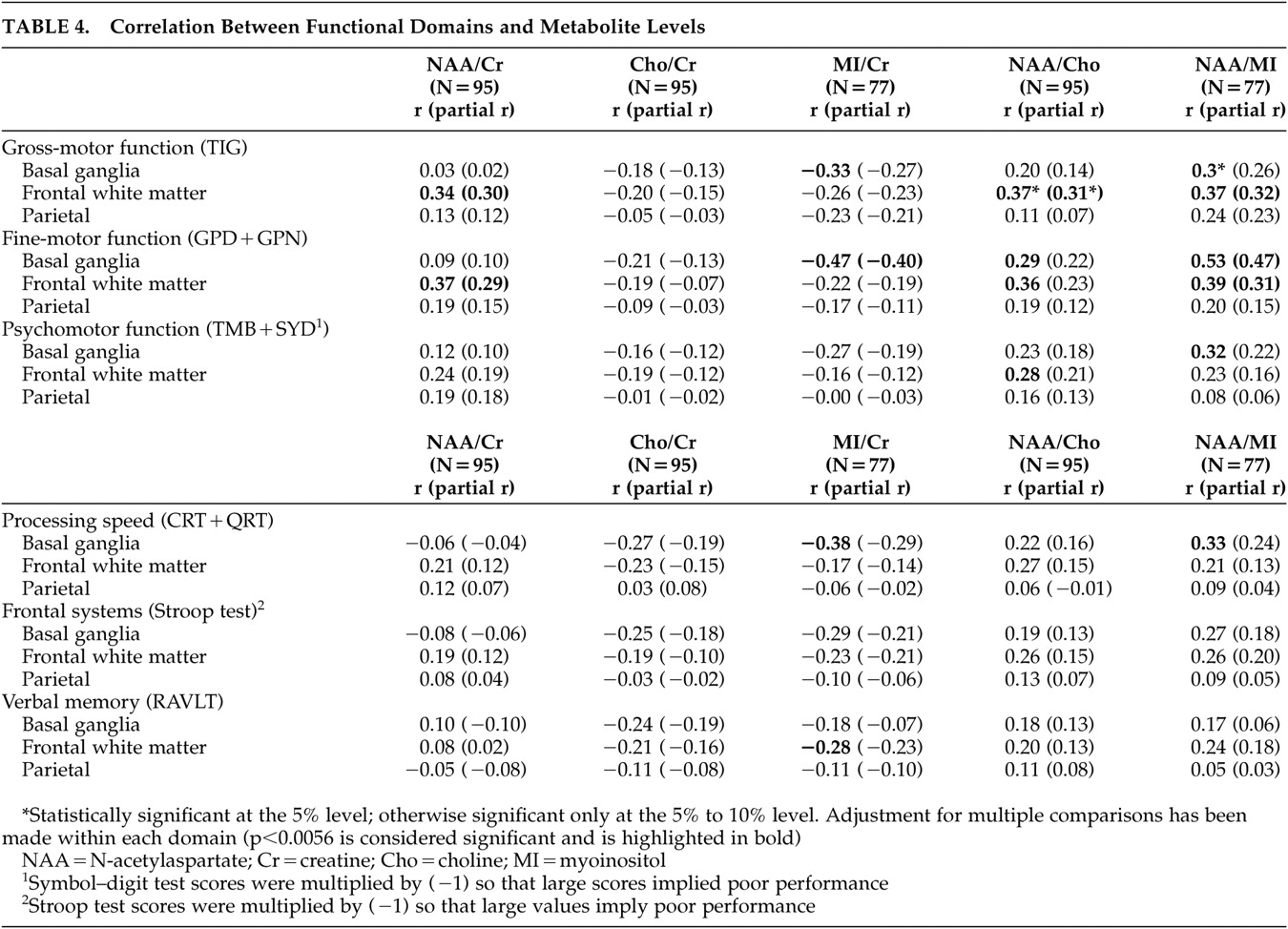
The present study extends the above findings by identifying the specificity of MRS/cognitive relationships across brain regions. In our study, impaired gross motor and fine motor skills were associated with increases in myoinositol/creatine, a presumed marker of gliosis, in the basal ganglia and with decreases in N-acetylaspartate/creatine, a marker of mature neurons, in the frontal white matter. It is of interest that we did not observe significant relationships between N-acetylaspartate/creatine levels in the basal ganglia and neuropsychological function. Studies conducted prior to the widespread use of HAART reported reduced N-acetylaspartate/creatine levels in the frontal gray matter among patients with ADC,
33 and one might expect similar results in the basal ganglia given the anatomical connections between these regions of the brain. However, N-acetylaspartate measured in the subcortical gray matter appears relatively intact in the earliest stages of neuropsychological compromise associated with HIV. For example, Chang et al.
36 reported no significant alterations in N-acetylaspartate in the basal ganglia among patients with mild neuropsychological dysfunction, and Meyerhoff et al.
52 observed significant reductions in N-acetylaspartate in the subcortical gray matter only among patients with severe neuropsychological impairments.
Most patients in the current study received an ADC stage score of 1, consistent with mild to moderate neuropsychological impairment. As such, our findings suggest that increasing inflammatory change or membrane damage in the basal ganglia contributes to neuropsychological impairment independent of the effects of a decrease in N-acetylaspartate/creatine, at least among patients with less severe ADC who are on stable treatment. Future studies will be needed to examine these MRS indices in multiple subcortical and cortical regions (including the frontal lobe gray matter) in order to delineate the relationships between changes in metabolite ratios and neuropsychological function across these defined brain circuits. In addition, examination of metabolite concentrations in these brain regions may be useful as previous work has demonstrated alterations in multiple metabolites in patients with HIV, including the reference metabolite creatine, and these alterations could influence the ratio values and possibly mask the effects of changes in individual metabolites (e.g., reductions in N-acetylaspartate).
36,
53Surprisingly, as both choline and myoinositol are markers of glial cell function and choline/creatine is increased in HIV-infected individuals, we found only weak associations with choline/creatine in the frontal white matter and basal ganglia and neuropsychological performance. Changes in myoinositol may thus reflect metabolic events not measured by choline. Together these findings suggest that abnormalities in myoinositol/creatine and N-acetylaspartate/creatine may provide the primary metabolic substrates underlying neuropsychological dysfunction among HIV-infected individuals. The observation that correlations were generally higher with measures of pure motor function and processing speed as opposed to psychomotor function and verbal memory further support earlier observations that impairment in these domains comprises one of the more salient manifestations of ADC.
2 –
11The current findings support the hypothesis that HIV-associated cognitive impairment is associated with dysfunction of subcortical brain structures and white matter.
2 –
11 Assuming that any disruption of subcortical function results in concomitant disruption of frontal-subcortical circuits, it is reasonable to conclude that frontal measures, including measures of psychomotor function, would tend to correlate both with basal ganglia dysfunction (the presumed site of damage) as well as interconnected white matter. In fact, psychomotor function as measured by Symbol Digit and Trail-Making Test, Part B correlated with metabolites in the basal ganglia and frontal white matter.
The relative lack of correlations between neuropsychological function and MRS indices in the parietal cortex is consistent with models of HIV-related brain injury.
14,
15 The most parsimonious explanation for this observation is that cortical abnormalities may be most significant among individuals with more advanced cognitive impairment. Alternatively, certain functional relationships may be localized to cortical regions other than the parietal cortex. The neuropsychological battery administered in the present study was not particularly sensitive to cognitive functions mediated by the parietal cortex, and therefore the lack of significant associations between the MRS indices from the parietal cortex and neuropsychological performance may reflect this bias. However, the parietal cortex is involved in attentional processes, among other tasks, and there is evidence of disrupted attentional networks among patients with HIV.
54 Future studies that employ more selective measures of attention will be required to define these relationships with greater certainty. Nevertheless, to the extent that brain metabolite abnormalities in the parietal cortex represent general cortical disease involvement, the absence of a significant relationship between MRS indices in the parietal cortex and neuropsychological performance emphasize the specificity of subcortical brain regions to neuropsychological impairment in HIV. As noted previously, this issue will be clarified with greater certainty in future studies that examine MRS indices in multiple brain regions, including the frontal gray matter.
Previous studies have revealed that the expression of host factors (inducible nitric oxide synthase, TNF-α) and viral proteins in the basal ganglia are predictive of earlier stages of ADC, while increasing expression of these factors in both the basal ganglia and white matter correlate with more advanced stages of cognitive impairment.
14,
15 It is of interest then that performance in only three domains of function, fine motor (Grooved Pegboard), gross motor function (Timed Gait) and information processing speed (CalCAP), was strongly associated with myoinositol/creatine in the basal ganglia, whereas fine and gross motor function in addition to psychomotor function (Trail-Making Test, Part B and Symbol Digit) correlated with metabolite changes in the frontal white matter. Metabolite changes in the basal ganglia may thus provide a sensitive marker of early neuropsychological impairment, while the subtests related to these changes may prove useful in its assessment.
Overall, our results reinforce the notion that HIV-associated neuropsychological impairment is associated with region-specific changes in the brain. Future studies will need to determine the long-term benefits of HAART on cerebral and cognitive function in the setting of chronic HIV infection. Additional studies are needed to elucidate the mechanisms that underlie the evolution of these neural changes in the setting of HAART and advanced disease, as well as the possibility to improve cognitive function with adjunctive pharmacological agents.
Acknowledgments
This study was supported by the AIDS Clinical Trials Group (NIAID) and grants NS36524 (NINDS), AI38855 (NIAID), and NIMH grant R03MH60565.
The MRS Consortium was established by the NINDS grant R01-NS-36524 to Dr. Navia. We thank Diane Rausch, Program in AIDS, NIMH for support of the ACTG 301 study. Normative data were collected by the Multicenter AIDS Cohort Study (MACS) with centers (principal investigators) at the Johns Hopkins University and Bloomberg School of Public Health (Joseph B. Margolick, Lisa Jacobson), Howard Brown Health Center and Northwestern University Medical School (John Phair), University of California, Los Angeles (Roger Detels, Beth Jamieson), and University of Pittsburgh (Charles Rinaldo). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute. UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041. The MACS web site is located at http://www.statepi.jhsph.edu/macs.html. Both ACTG 301 and 700 were undertaken with support by the AIDS Clinical Trials Group (ACTG) under grant U01-AI-38858 from the NIAID. Dr. Yiannoutsos’ research was partially supported by the Statistics and Data Analysis Center through NIAID grant U01-AI-38855 and from grant R03-MH-60565 from NIMH. Dr. Miller is the author and distributor of the CALCAP reaction time program and has a financial interest in this software. Dr. Paul’s research was partially supported by grant K23MH65857.