A mong adults infected with HIV, the rate of major depression is nearly two times higher than in HIV-seronegative adults
1 with a history of intravenous drug use and a previous history of major depression, and is associated with a substantial increased risk for at least one post-HIV major depressive episode.
2 –
3 It has been posited that in some individuals living with HIV, depression is secondary to the neurotropic effects of the virus on brain structures.
4 As it is well recognized that lesions in specific neuroanatomic structures due to disease or insult, such as those resulting from a stroke or brain tumor, may contribute to the development of secondary depression, and that HIV can directly or indirectly affect the integrity of the cerebrum,
5 the notion of a secondary depression in HIV is certainly tenable. To verify the secondary nature of HIV-associated depression, studies have generally sought to correlate depression severity with HIV-associated cognitive deficits.
6 –
7 Yet, studies have consistently demonstrated that HIV-associated cognitive dysfunction is not associated with either depression severity
6 –
9 or incidence,
10 leading some to conclude that there is no evidence of a secondary depression in HIV.
6 –
7 Among adults with mood dysfunction, volumetric reductions in cortical and subcortical regions include the frontal cortex, anterior cingulate, hippocampus, and basal ganglia.
11 –
15 Within these regions, a left-sided, anterior predominance of alterations has been reported,
12 although this has not been universal.
16 In addition to volumetric reductions, alterations in white matter microstructure, as evidenced by reductions in fractional anisotropy, have been identified within the dorsolateral prefrontal cortex, anterior cingulate, left lateral occipitotemporal region, parietal lobe, and parahippocampal gyrus.
17 –
19 Finally, functional MRI (fMRI) techniques have revealed a widespread pattern of altered activation within the left, right, and bilateral hemispheres in areas such as the insula, hippocampus, amygdala, thalamus, temporoparietal cortex, and basal ganglia.
20 –
24Overall, the abnormalities in structure and function have implicated the limbic-thalamic-cortical circuit, involving the amygdala, medial thalamus, and prefrontal cortex, and the limbic-cortical-striatal-pallidal-thalamic circuit, consisting of the component of the limbic-thalamic-cortical circuit and the connections to the striatum and pallidum.
20,
25 –
26 Within the limbic-cortical-striatal-pallidal-thalamic/limbic-thalamic-cortical circuit, recent studies have demonstrated that discrete components of depression (e.g., apathy, anxiety, and sleep disturbance) relate to unique neuroanatomic structures within this network,
21 –
23 highlighting the multifactorial nature of depression.
It is well documented that HIV is associated with global and regional brain atrophy,
5 particularly with advanced HIV disease. Regional increases and decreases in fractional anisotropy and mean diffusivity have been reported throughout the cerebrum.
27 –
29 Importantly, abnormal anisotropy has been reported in the frontal white matter of patients with HIV even though no white matter abnormalities were evident on T2-weighted MRI, suggesting that the diffusion tensor imaging technique is sensitive to early HIV-associated pathologic change.
28Given that the very brain structures and neural circuits known to be associated with mood dysfunction in adults without HIV infection comprise putative neural networks and structures known to be vulnerable to HIV disease, it may be that HIV infection contributes to the development, maintenance, and exacerbation of mood dysfunction by altering the integrity of specific brain structures and neural networks associated with mood regulation. Yet, to date, we are aware of only two studies that have utilized structural neuroimaging to specifically examine the relationship between HIV-associated brain structural alterations and depressed mood.
30 –
31 While basal ganglia atrophy is associated with HIV infection
5 and with major depression in adults without HIV,
15 Davidson and colleagues
30 failed to find an association between basal ganglia atrophy and depression in adults with HIV. However, in a more recent study, apathy, a discrete component of depression, was associated with decreased volume of the nucleus accumbens.
31 Given the general reliance upon correlational relationships between depression and HIV-associated cognitive dysfunction to support/refute the secondary nature of depression in HIV,
6 –
7 it may be that the failure to find a direct correlation is due to the failure to consider the multifactorial nature of depression.
In this study, utilizing in vivo diffusion tensor imaging, we compared radiologically defined normal appearing white matter (NAWM) microstructural integrity in HIV-seropositive (HIV+) adults with and without prominent self-reported mood dysfunction, hypothesizing that HIV+ adults with depressive symptoms would demonstrate alterations within regions associated with mood regulation. Additionally, based on recent findings,
21 –
23 we examined the relationship between discrete symptom components of depression and NAWM fractional anisotropy microstructure, hypothesizing that the discrete symptom components would differentially relate to alterations in NAWM fractional anisotropy microstructure.
METHODS
Participants
Thirty HIV+ community dwelling adults were enrolled. The participants were categorized as presenting with significant depressive symptoms if they attained a score of greater than 14 on the 21-item Beck Depression Inventory-II (BDI-II).
32 Fifteen HIV+ adults (10 men and five women) reported significant depressive symptoms (HIV-D) and 15 HIV+ adults (10 men and five women) denied significant depressive symptoms (HIV-N).
Each participant was right-handed, received their primary education in English, and demonstrated an estimated Full Scale IQ score ≥70 based on the Wechsler Adult Reading Test.
33 Exclusionary criteria included: a history of closed head injury with loss of consciousness greater than 30 minutes; a previous diagnosis of dementia, schizophrenia, or nonaffective psychotic disorder; substance abuse within the past 6 months based on self-report; a history of stroke or seizure disorder; and/or having contraindications to MRI scanning, including the presence of certain types of surgically implanted devices (e.g., pacemakers), metal clips, prostheses, or other ferromagnetic materials/fragments in high risk regions of the body and resting tremor.
Participants were randomly recruited from the Infectious Disease clinics at Rush University Medical Center, the Ruth M. Rothstein CORE Center, and John H. Stroger, Jr. Hospital of Cook County, as well as by word of mouth among enrolled participants. All subjects provided written informed consent as approved by the Institutional Review Boards at Rush University Medical Center and Cook County Bureau of Health Services.
Behavioral Measures
Each participant underwent a detailed neurobehavioral assessment within 4 weeks of their MRI scan, which involved a neurological evaluation, mental status testing using the Mini-Mental State Examination (MMSE),
34 and a psychosocial interview. The neurological evaluation was completed by a board-certified neurologist (REB); a board-certified neuropsychologist (CAS) completed the neurobehavioral assessment.
Past history of major depression was determined by self-report. Current severity of depressive symptoms was assessed with the 21-item BDI-II within 4 weeks of completing the MRI protocol. With total scores ranging from 0 to 63, scores of 14 or greater are often used to indicate clinically significant depressive symptoms.
32 Additionally, all participants completed the Chicago Multiscale Depression Inventory,
35 a 42-item self-report rating inventory that was designed and validated to assess discrete components of depression, including self-evaluation, mood, and neurovegetative symptoms. Given that neurovegetative symptoms, such as fatigue and motor slowing, are associated with HIV and that the overlapping symptoms of HIV disease and somatic depression can inflate scores on the BDI,
36 the Chicago Inventory subscales (mood, evaluative, and vegetative) have been previously utilized to assess mood disturbance among patients with HIV.
37Imaging Procedures
MRI was performed on a 1.5T scanner (GE Medical Systems, Milwaukee) equipped with fast gradient Horizon Echospeed upgrades (Rev. 11.4). Twenty-six diffusion tensor imaging volumes were obtained for each participant, including 24 volumes with diffusion gradients applied along noncollinear directions with three repetitions (b=800 sec/mm 2 ) and two volumes without diffusion weighting (b=0). Each volume was composed of 38 contiguous axial slices acquired using a single-shot echoplanar diffusion-weighted imaging method (TR 12100, TE 97, gradient duration δ=20ms, acquisition matrix 128×128, FOV=250, slice thickness =3 mm 0 gap).
Postacquisition processing of diffusion tensor images followed established procedures,
38 resulting in whole-brain T2 (0 diffusion weighted) and fractional anisotropy volumes. Briefly, whole-brain volumes were imported into Statistical Parametric Mapping software (SPM2). We limited fractional anisotropy values in NAWM by creating individual subject mask volumes to exclude voxels representing white matter abnormalities based on T2 signal, which we defined based on a probability of greater than 0.80 for white matter classification from the white matter T2 segmented image. The individual NAWM masks were then applied to individual subject fractional anisotropy maps and smoothed with a 6 mm full width at half maximum Gaussian filter.
Associations between depressive symptoms on the BDI-II, Chicago Multiscale Depression Inventory subscales, and average whole-brain NAWM fractional anisotropy were evaluated in STATISTICA (StatSoft, Tulsa). Regional associations between depressive symptoms and fractional anisotropy were evaluated using voxel-wise analysis of covariance and regression analyses in SPM2 entering age, drug use history, and previous depression diagnosis as covariates of no interest. For average whole-brain NAWM fractional anisotropy value comparisons, significance was determined with a p value of less than 0.05. For all voxel-wise comparisons, significance was determined with a p value of less than 0.001 with a contiguous cluster size of 20 voxels.
RESULTS
Sociodemographic and Medical Data
No differences in age, education, or general intellectual abilities between the two groups were identified (
Table 1 ). By self-report, 16 subjects (eight HIV-D and eight HIV-N) had been diagnosed with mood disorder in the past. Three HIV-D and one HIV-N participants were currently prescribed mood-stabilizing medication. Nine HIV-D and six HIV-N subjects reported a history of drug or alcohol abuse; drug use included cocaine (HIV-D=8, HIV-N=4), heroin (HIV-D=5, HIV-N=4), marijuana (HIV-D=6, HIV-N=2), and alcohol (HIV-D=1, HIV-N=4). All participants denied a history of alcohol or drug abuse within the past 6 months; the last reported drug use ranged from 12 to 192 months for the HIV-D group and 12 to 252 months for the HIV-N group. Urine toxicology was not completed at the time of neuroimaging. There were no significant differences in CD4, viral load, or measures of mood status between the subjects with or without a history of drug or alcohol abuse. Eleven HIV-D and 15 HIV-N subjects were currently taking antiretroviral medications. Eight HIV-D and four HIV-N subjects had a detectable viral load (>75 copies); there were no significant differences in mood state between those with and those without detectable viral load. There were no significant differences in plasma CD4 or percent CD4 lymphocytes between HIV-D and HIV-N participants. Correlations between markers of immunologic and virologic health (CD4, percent CD4, viral load) and all measures of mood status were nonsignificant. The HIV-N subjects presented with significantly longer duration of known HIV infection, which was defined as the time since their first HIV RNA positive test (t=2.95, df=1, 28, p=0.006). Review of available medical records indicated that 10 participants (six HIV-D and four HIV-N) were hepatitis C virus antibody positive; measures of current hepatitis C virus viremia were not available.
Consistent with the group dichotomization based on the BDI, the HIV-D group attained significantly higher scores on the Chicago Multiscale Depression Inventory subscales of mood (t=4.24, df=1, 28, p<0.001) and evaluative (t=2.7, df=1, 28, p=0.01). There was a trend for the HIV-D group to report more vegetative symptoms (t=2.00, df=1, 28, p=0.06).
Whole-Brain NAWM Fractional Anisotropy Associations
The HIV-D and HIV-N participants did not differ in whole-brain NAWM fractional anisotropy. A lower percentage of CD4 cells correlated with a decrease in NAWM fractional anisotropy (r=0.53, p=0.003). NAWM fractional anisotropy was not associated with current CD4 cell count or viral load (log10). There was a trend for longer duration of known HIV infection to be associated with decreased NAWM fractional anisotropy (r=−0.35, p=0.06); this trend disappeared after adding age as a covariate of no interest (sr=−0.17, p=nsec). Correlations between whole-brain NAWM fractional anisotropy and all measures of mood status were nonsignificant.
Regional Alterations Between HIV-D and HIV-N Groups
After entering age, drug use history, and previous diagnosis of mood dysfunction as covariates of no interest, regional differences in NAWM fractional anisotropy between HIV+ adults with significant depressive symptoms (HIV-D) and HIV+ adults without depressive symptoms (HIV-N) were evaluated. HIV+ adults with increased depressive symptoms evidenced significantly higher NAWM fractional anisotropy within the left thalamus (Z=4.46, cluster size=96, Talairach x/y/z: −14, −23, 12), left superior temporal white matter (Z=4.35, cluster size=55, Talairach x/y/z: −36, −36, 15), left superior frontal lobe (Z=3.68, cluster size=21, Talairach x/y/z: −30, −23, 35), and right cingulate (Z=3.65, cluster size=35, Talairach x/y/z: 10, 19, 40) (see
Figure 1 ). There were no regions in which the HIV-D group presented with significantly lower NAWM fractional anisotropy.
Regional Associations With Discrete Components of Depression
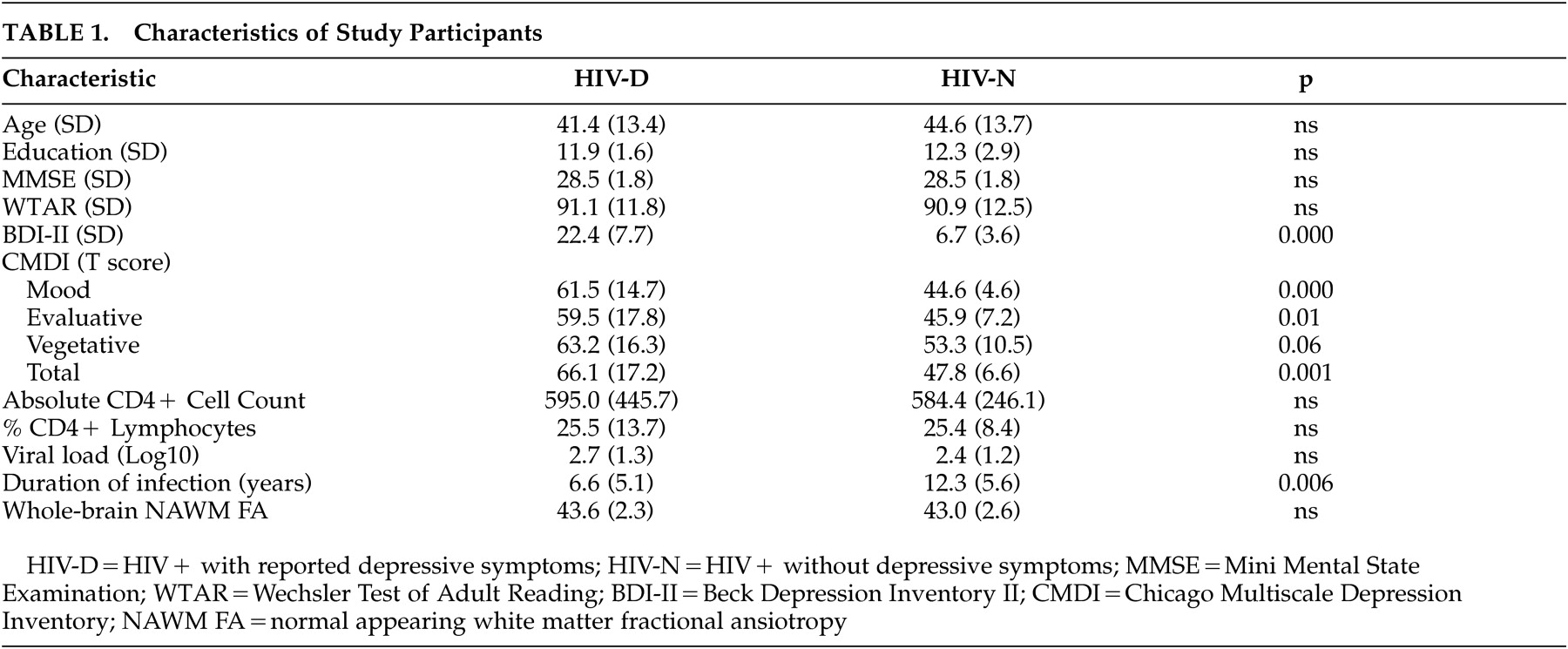
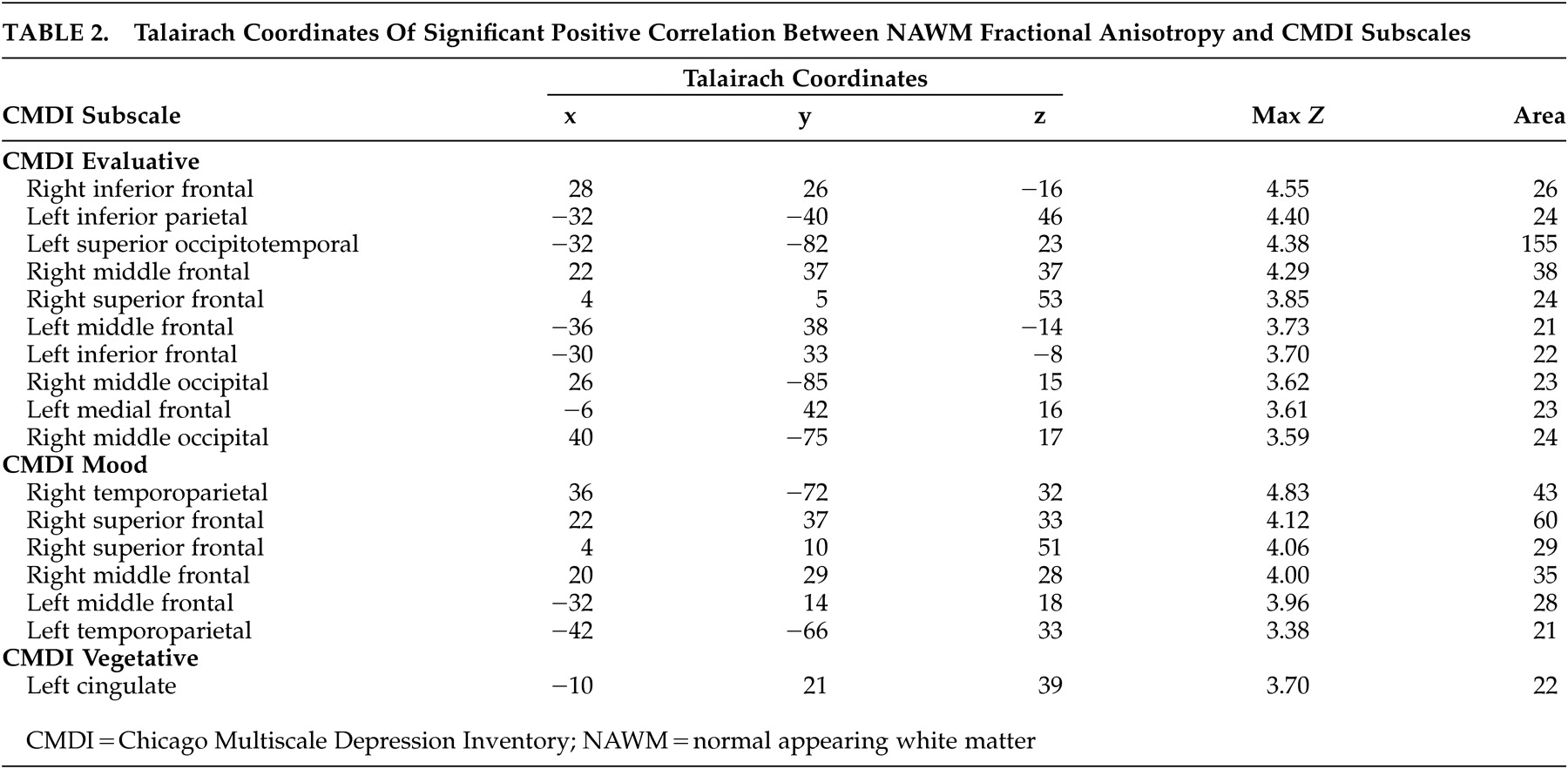
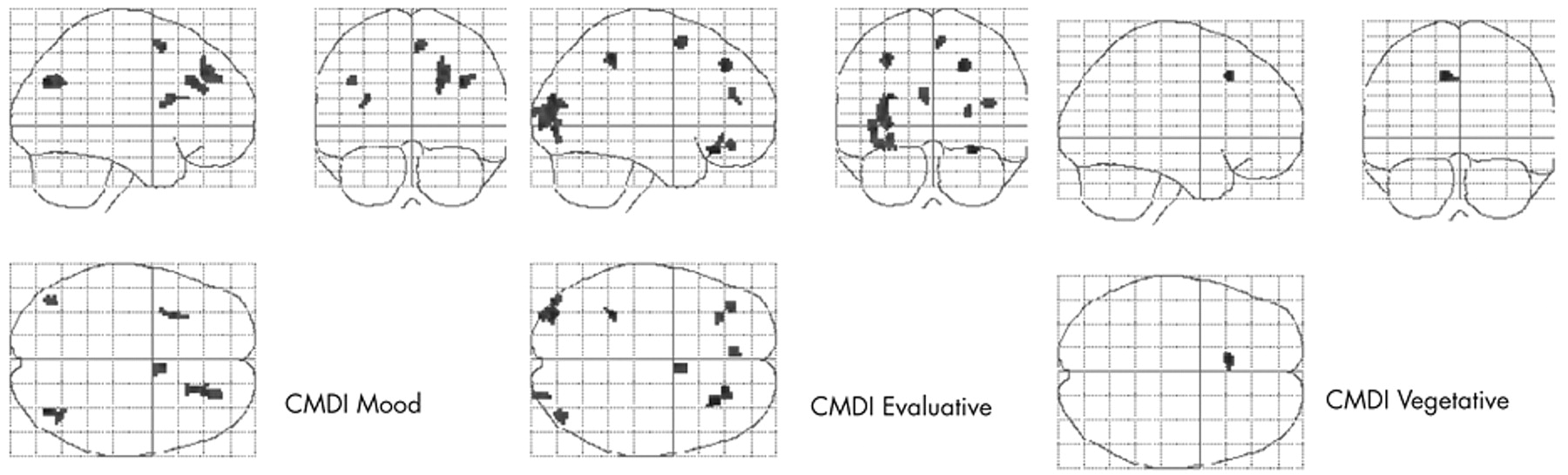
Within the HIV-D group only, after controlling for age, drug use history, and depression history, regionally distinct correlation patterns between NAWM fractional anisotropy and the three Chicago Multiscale Depression Inventory subscales were found (see
Table 2 and
Figure 2 ). For the Chicago Inventory evaluative subscale, increased symptoms correlated with increased NAWM fractional anisotropy within 380 voxels. Significant positive associations between the Chicago Inventory mood subscale and NAWM fractional anisotropy were identified within a predominately anterior distribution covering 216 voxels. Finally, the Chicago Inventory vegetative subscale was associated with a single region of increased NAWM fractional anisotropy covering 22 voxels. For all discrete components of the Chicago Multiscale Depression Inventory, there were no significant regions where increased depressive symptoms correlated with decreased NAWM fractional anisotropy.
DISCUSSION
In the current study, we evaluated differences in normal appearing white matter (NAWM) fractional anisotropy in HIV+ adults with and without significant depressive symptoms. As hypothesized, HIV+ adults with significant depressive symptoms evidenced altered NAWM fractional anisotropy within regions previously associated with mood regulation, including regions within the right anterior cingulate and left thalamus. No regions of decreased NAWM fractional anisotropy were revealed. Additionally as hypothesized, discrete depressive symptoms were associated with distinct regional alterations in NAWM fractional anisotropy. While the distribution of altered NAWM fractional anisotropy tended to be anterior, consistent with previous research,
18,
21,
23 –
24 alterations in the temporoparietal/occipital regions were shown to relate to discrete mood symptoms.
In adults with major depression, morphological alterations have been reported in cortical and subcortical structures, including the basal ganglia, parahippocampal cortex, amygdala, orbitofrontal cortex, and anterior cingulate gyrus.
25 –
26 Alterations in functional neural networks, including areas in the bilateral frontal cortex, parietal cortex, caudate head, and thalamus, have also been reported.
20 –
24 As many of these structures comprise putative neural networks known to be vulnerable to HIV disease, depression secondary to HIV disease as postulated by Heisman et al.
4 is certainly tenable. Yet, secondary HIV-related depression has generally been dismissed.
6 –
7 Recently, a discrete component of depression, apathy, which is associated with cognitive dysfunction,
39 has been associated with atrophy of the nucleus accumbens in patients with HIV,
31 suggesting a possible link between HIV-associated regional atrophy, cognitive dysfunction, and mood dysfunction. Our finding that adults with HIV and increased self-reported depressive symptoms evidence regional alterations in white matter microstructure offers additional support for depression secondary to HIV infection. Additionally, our finding that the separate components of depressive symptoms are related to discrete neuroanatomic regions of increased NAWM fractional anisotropy highlights the multifactorial nature of depression and suggests that specific phenomenology is associated with discrete white matter alterations.
Previous studies have reported that major depression is associated with alterations in white matter microstructure, as evidenced by reductions in fractional anisotropy (fractional anisotropy), within the dorsolateral prefrontal cortex, anterior cingulate, left lateral occipitotemporal region, parietal lobe, and parahippocampal gyrus.
17 –
19 In the current study, while alterations within these regions were identified, rather than reductions in regional fractional anisotropy, both the discrete components of depression and the regional differences between those with and without depressive symptoms were associated with regional increases in NAWM fractional anisotropy. While this is a surprising and unexpected finding given that decreases in fractional anisotropy are generally thought to reflect the degradation of the microstructural white matter integrity, increases in anisotropy, which may reflect a decrease in the complexity of white matter crossing fibers,
40 have been reported in HIV.
28 With this, the current results may reflect discrete, regional alterations in white matter complexity in patients with HIV. Exploratory analyses comparing the HIV+ adults with and without depressive symptoms utilizing a lower significance threshold (p=0.005) revealed that HIV+ adults with depressive symptoms evidenced regional decreases in NAWM fractional anisotropy within the left orbitofrontal region; yet, given our concern to limit type I errors, we specifically selected the more conservative significance level, recognizing that we may fail to identify true, regionally significant differences.
Importantly, it is not clear from this cross-sectional study whether the identified regional alterations are a direct result of HIV infection or are attributable to other comorbid factors that have been associated with increased risk for HIV-related disease, such as nadir CD4, hepatitis C virus co-infection, or past or current alcohol/drug abuse. Further, while the number of subjects reporting a previous history of major depression was equally distributed between the two groups, as a history of at least one pre-HIV major depressive episode has been associated with an increased risk of developing major depression following HIV infection,
2 –
3 it may be that the identified alterations are associated with pre-HIV alterations in the neural matrix. Longitudinal studies of white matter alterations in HIV+ patients with and without a previous history of major depression are necessary in this regard. Nevertheless, this study reveals that discrete regions of altered neural complexity are associated with current self-reported mood disruption, which adds additional support for secondary HIV-associated mood dysfunction.
To our knowledge, this is the first study that maps correlates of white matter integrity and self-reported severity of depressed mood in patients living with HIV/AIDS. Importantly, the sample size is relatively small, and the results need to be replicated in independent samples, as our sample size prohibited inclusion of all possible covariates (e.g., nadir CD4, hepatitis C virus status, frequency/duration/quantity of illicit drug use). However, our sample size was sufficiently large to account for the differences in age, reported substance abuse history, and reported depression history, which may independently alter white matter integrity. We had a limited number of subjects who were not on HIV therapy, so we could not investigate potential anisotropic differences of white matter integrity in treated versus untreated patients. The regional differences in anisotropy were based upon self-reported depressive symptoms; replication studies utilizing larger samples and formal diagnostic clinical interviews for the diagnosis of major depression disorder will be vital. Relationships between the discrete regional alterations associated with depressive symptoms and their impact on neurocognition remain to be evaluated.
Despite these potential limitations, our results may help reconcile previous conflicting reports regarding secondary HIV-associated mood dysfunction. Mood regulation is known to be associated with a broad neural network, and our results suggest that regional decreases in neural complexity contribute to discrete depressive symptoms, supporting not only the circuitry model,
20 but also the notion that mood dysfunction is multifactorial with alterations in different brain structures being associated with the discrete components of depression.
Acknowledgments
Dr. Stebbins has received research funding, consulting fees, or lecture fees from Kinetics Foundation, Berlex Pharmaceuticals, Michael J. Fox Foundation, Rayman Family Fund, and Schering Pharmaceuticals. Dr. Kessler has received research funding, consulting fees, or lecture fees from Abbott Laboratories, Merck, GlaxoSmithKline, Bristol Myers-Squibb, Boehringer-Ingelheim, TiboTec, TheraTechnologies, Gilead, Virco, and Pfizer. Dr. Adeyemi has received research funding, consulting fees, or lecture fees from Abbott Laboratories, GlaxoSmithKline, TiboTec, and Idenix. Drs. Bammer and Moseley have received research funding from GE Healthcare and Endius, Inc.
Data in this manuscript were collected at the Ruth M. Rothstein CORE Center for the Prevention, Care and Research of Infectious Diseases, a joint venture of the Cook County Bureau of Health Services and Rush University Medical Center with support from the National Institute of Aging (R21 AG23491, to Dr. Smith); and the Rush University Medical Center-Cook County Collaborative Grant (to Dr. Smith).