M ethamphetamine use and dependence is a growing public health concern in the United States.
1 It is only in the last decade, however, that researchers have examined the effects of methamphetamine on the human brain. For example, the neurobiological abnormalities associated with long-term, high-dose methamphetamine exposure have been described using various neuroimaging
2 –
5 and electrophysiological
6 techniques. The functional consequences of long-term, high-dose methamphetamine exposure are also reasonably well-established, and include marked deficits on measures of episodic memory.
7 To our knowledge, only two studies have examined episodic memory deficits and neurobiological abnormalities in the same set of methamphetamine-dependent individuals. One study of 15 subjects used [
11 C]d-
threo -methylphenidate to image the dopamine transporter (DAT) and showed that reduced levels of striatal DAT were associated with impairment on the Rey Auditory Verbal Learning Test.
2 In another study of 22 subjects, high-resolution MRI revealed that reduced hippocampal volume was associated with poorer performance on a memory test that was constructed specifically for use in that laboratory.
5In this study we investigated the link between memory impairment and underlying brain function using quantitative electroencephalography (QEEG) in recently abstinent, long-term, high-dose methamphetamine users. We hypothesized that poorer performance on episodic memory tests would be associated with increased theta power, an index of neurobiological abnormality, based on previous work that has demonstrated this association
8 and other studies showing that methamphetamine-dependent individuals demonstrated increased theta absolute power.
6METHODS
Participants
Nineteen volunteers were recruited from the community through advertisements in local newspapers. Participants included nine non-treatment-seeking, methamphetamine-dependent volunteers and 10 non-drug-using comparison subjects. The methamphetamine-dependent participants met DSM-IV criteria for methamphetamine dependence using the SCID.
9 None met criteria for psychosis, mood disorder, or dependence on other drugs except nicotine, though they used alcohol and marijuana without meeting dependence criteria. The comparison subjects never used stimulants nor met criteria for abuse of any drug except for nicotine. Moreover, based on the Structured Clinical Interview for DSM-IV (SCID), comparison subjects did not meet the diagnostic criteria for other psychopathology. Potential participants were excluded for a history of stroke, traumatic brain injury (or loss of consciousness greater than 20 minutes), epilepsy, attention deficit disorder, or for testing HIV seropositive. Participants provided written informed consent to enroll in the study after being apprised of the study risks, which was approved by the UCLA Human Subjects Protection Committee. They were reimbursed for their participation.
Methamphetamine-dependent individuals were long-term, high-dose users who ingested, on average, at least 2.0 grams of methamphetamine per week for the 6 months prior to the study. Seven methamphetamine users smoked the drug, two snorted it, and none reported intravenous use.
Methamphetamine-dependent individuals resided in the General Clinical Research Center at UCLA for the duration of the study to ensure abstinence. Upon admission, they tested positive for methamphetamine, which demonstrated recent use of the drug. Daily urine testing confirmed their abstinence during enrollment.
QEEG Assessment
EEGs were recorded on day four of the study. The procedures used and results have been described previously for this sample.
6 Briefly, participants were evaluated while resting in the eyes-closed, maximally alert state in a sound-attenuated room. An electrode cap (ElectroCap, Eaton, Ohio) with 35 recording electrodes was placed according to the International 10–20 System and was used to record EEG data. At least 20 minutes of EEG activity were recorded. A technician reviewed each EEG tracing and selected the first 20–32 seconds of artifact-free data for processing, with selections confirmed by a second technician. Subjects’ identities were masked. Data were collected using a Pz referential montage and digitized at 256 samples/channel/second by the QND system (bandpass filtered 0.3 Hz–70 Hz). A fast Fourier transform was used to calculate power in four frequency bands (0.5–4 Hz—delta; 4–8 Hz—theta; 8–12 Hz—alpha; and 12–20 Hz—beta). EEG data were log-transformed prior to analysis to reduce skew.
Neurocognitive Assessment
Study participants were administered a 2.5 hour battery of tests, which included Controlled Oral Word Association
10 ; the letter-number sequencing and the visual memory span subtests of the Wechsler Memory Scale-III
11 ; the Ruff Figural Fluency Test
12 ; the Rey Auditory Verbal Learning Test
13 ; the logical memory subtest of the WMS-III; the Rey Complex Figure Test
14 ; the Stroop Color-Word Test
15 ; the Symbol Digit Modalities Test
16 ; and the Trail Making test, parts A and B.
10 The screening measures included the Beck Depression Inventory (BDI)
17 and the National Adult Reading Test,
18 which served as an estimate of premorbid intellectual functioning.
Earlier findings from our laboratory,
19 as well as others,
2,
20 have shown that long-term, high-dose methamphetamine use is associated with impairment on measures of verbal and nonverbal episodic memory. For this reason, we selected the delayed recall subtests of the Rey Auditory Verbal Learning Test and the Rey Complex Figure Test as indices of verbal and nonverbal learning and memory, respectively.
13,
14Statistical Analyses
A previously used approach to minimize the likelihood of type I error was applied.
21 For example, mean power across all scalp electrode sites for each band was calculated. Additionally, nonparametric analyses, Spearman correlation coefficients, were used to calculate the degree of association between performance on the memory tests and the QEEG measures and to minimize the probability that outliers or skewed data distributions would affect the results. Because it was hypothesized
a priori that neurocognitive impairment was associated with increased mean power in low-frequency QEEG bands, one-tailed tests of significance were used.
RESULTS
The participants were comparable with regard to age (comparison subjects: mean age=35.7 years old, SD=7.1 versus methamphetamine users: mean age=33.2, SD=7.7), gender (comparison subjects: eight men, two women; methamphetamine users: seven men, two women), estimated level of premorbid intellectual functioning as measured on the National Adult Reading Test (comparison subjects: mean=108.6, SD=7.4; methamphetamine users: mean=105.2, SD=7.6), and self-reported level of depressive symptoms as measured by the BDI (comparison subjects: mean=9.5, SD=3.2; methamphetamine users: mean=7.8, SD=6.0). Within each group, demographic variables were not correlated with performance on the neurocognitive measures or QEEG indices. Mean power in the delta (comparison subjects: mean=1.2, SD=0.2 versus methamphetamine users: mean=1.3, SD=0.3) and theta (comparison subjects: mean=1.2, SD=0.2 versus methamphetamine users: mean=1.3, SD=0.3) bands differed significantly between the groups (p<0.01); in contrast, the alpha and beta bands did not differ significantly (p>0.05).
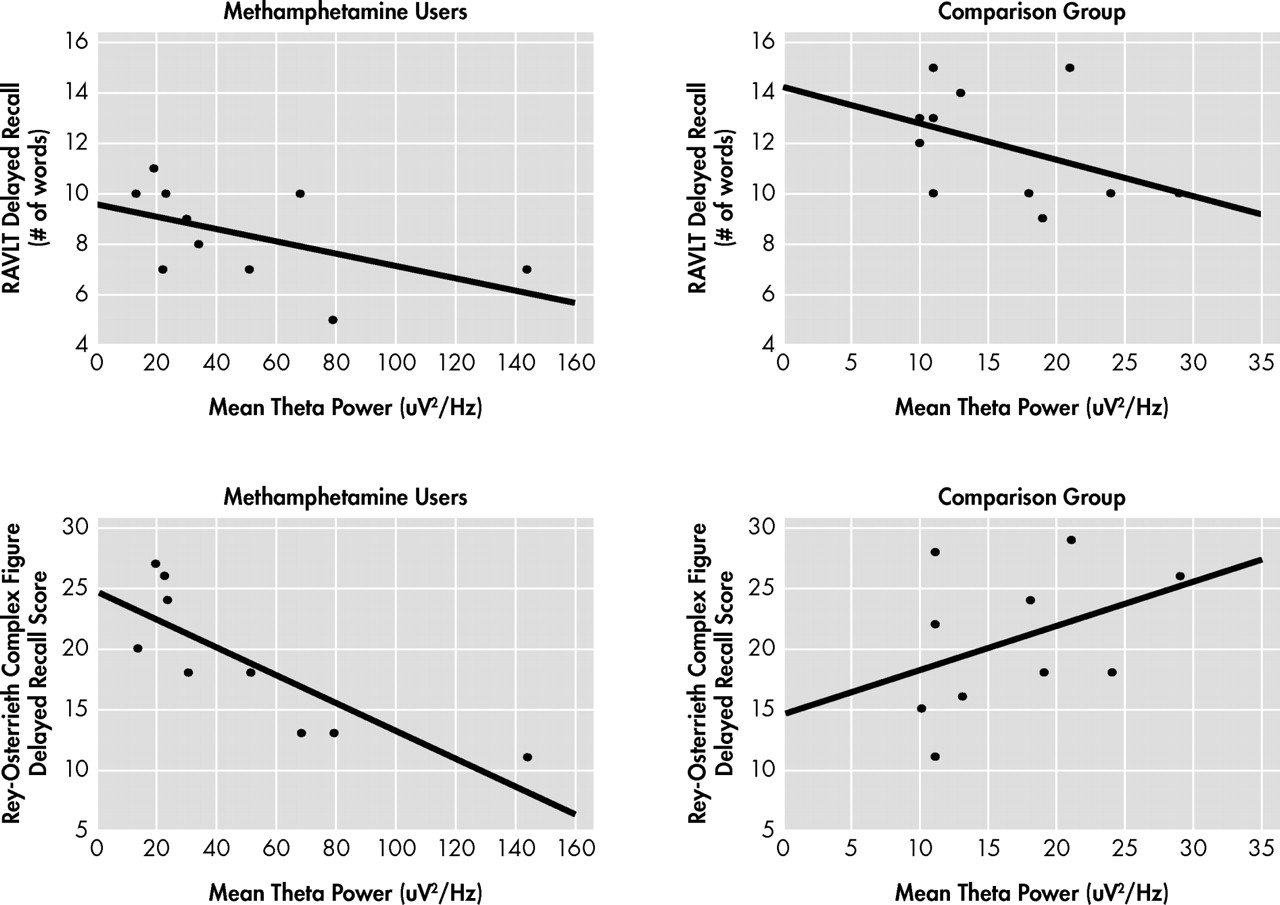
Performance on the neurocognitive measures was not correlated with the QEEG indices in the comparison group. In the methamphetamine-dependent group, mean power in the delta band was not correlated with performance on either memory test (p>0.10; data not shown). In contrast, for methamphetamine users, as shown in
Figure 1, increased mean power in the theta band was significantly correlated with poorer performance on the delayed recall subtest of the Rey Auditory Verbal Learning Test (ρ=−0.62, df=
9, p<0.03, R
2 =0.38) and with poorer performance on the delayed recall subtest of the Rey Complex Figure Test (ρ=−0.90, df=9, p<0.01, R
2 =0.71).
DISCUSSION
Consistent with our hypothesis, we found that increased theta EEG power was associated with poorer performance on measures of memory in long-term, high-dose methamphetamine users, but not in comparison subjects. These results complement the findings from previous studies
2,
21 by demonstrating that electrophysiological abnormalities associated with methamphetamine dependence are likely to affect behavior in an observable and important manner (i.e., memory deficits) when users are not intoxicated. Furthermore, this association is consistent with that observed in other disorders; specifically, a growing literature links theta activity to information retrieval.
8 These sophisticated cognitive-EEG approaches involve recording of EEG during the performance of cognitive tasks. In this study, we recorded resting-state eyes-closed EEG, precluding the use of those informative approaches.
While these findings are encouraging, several limitations should be noted. The modest sample size prohibited the identification of factors moderating the association between neurocognition and QEEG power (e.g., patterns of drug use). Furthermore, participants were abstinent for only 4 days. Though the available research and the data on these subjects limit the likelihood that withdrawal symptoms affected theta oscillations and performance on the memory tests, longitudinal assessment of abstinent methamphetamine-dependent individuals will clarify whether these deficits resolve over time. Additionally, there was no relationship between theta power and performance on memory tests in comparison subjects. This finding was not interpreted to suggest that the association does not exist in individuals without psychopathology; rather, because comparison subjects are more likely to demonstrate a truncated range of scores on memory tests, a larger sample of comparison subjects would be required to see a significant correlation between these two biomarkers. Additionally, we did not examine relationships between memory and EEG power calculated using different approaches (e.g., relative power, coherence, etc.).
To our knowledge, the neurobiological abnormalities that underlie these QEEG abnormalities in methamphetamine-dependent individuals have not yet been identified; however, this relationship has been investigated in other cohorts. For example, in samples of healthy control subjects, alterations in serotonergic,
22 dopaminergic,
23 and cholinergic
24 function were associated with changes in theta function and neurocognition. These studies suggest an approach for determining the specific neurochemical changes that underlie theta and neurocognitive abnormalities in methamphetamine users. Future studies might investigate the association between these biomarkers and important functional outcomes, such as ability to remain abstinent and/or employment status.
Acknowledgments
This research was supported in part by grants from the NIH DA50038, DA00388, DA07272, MH01483, MH01165, and RR-00865.